Chapter I: The Various Interpretations of the Experiments which Reveal the Dissociation of matter
(1) The First Interpretation
The ether and matter form the two extreme limits of the series of things. Between these limits, far as they are from each other, there exist intermediate elements, of which the existence is now revealed by observation. None of the experiments I shall set forth, however, will show us the transformation of ether into material substances. It would require the disposal of colossal energy to effect such a condensation. But the converse transformation of matter into the ether, or into substances akin to the ether is, on the contrary, realizable, and can be realized by the dissociation of matter. It is in the discovery first of the cathode rays and then of the x-rays that are found the germs of our present theory of the dissociation of matter. This dissociation, whether spontaneous or induced, always reveals itself by the emission into space of effluves identical with the cathode and the x-rays. The assimilation of these two orders of phenomena, which for several years I was alone in maintaining, is today universally admitted.
The discovery of the cathode and of the x-rays which invariably accompany them, marks one of the most important stages of modern science. Without it, the theory of the dissociation of matter could never have been established; and without it, we should always have been ignorant that it is to this dissociation of matter that we owe phenomena long known in physics, but which had remained unexplained. Every one knows at the present time what the cathode rays are. If through a tube furnished with electrodes and exhausted to a high vacuum an electric current of sufficient tension be sent, the cathode emits rays which are projected in a straight line, which heat such bodies as they strike, and which are deviated by a magnet. The metallic cathode only serves to render the rays more abundant, since I have proved by experiment that with a Crookes’s tube without cathode or any trace of metallic matter whatever, exactly the same phenomena are observed.
The cathode rays are charged with electricity, and can traverse very thin metallic plates connected with the earth without losing their charge. Every time they strike an obstacle they immediately give rise to those peculiar rays termed x-rays, which differ from the cathode rays in not being deviated by a magnet, and pass through thick metallic plates capable of completely stopping the cathode rays. Both cathode and x-rays produce electricity in all bodies that they meet, whether they be gases or solid matter, and consequently render the air a conductor of electricity.
The first ideas of the nature of the cathode rays which were conceived were far different from those current today. Crookes, who first put in evidence the properties of these rays, attributed their action to the state of extreme rarification of the molecules of the gas when the vacuum had been carried very far. In this “ultra-gaseous” state, the rarified molecules represented, according to him, a peculiar state which can be described as a fourth state of matter. It was characterized by the fact that, no longer hindered in their course by the impact of the other molecules, the free trajectory of the rarified molecules lengthens to such a point that their reciprocal shock becomes of no importance compared with their whole course. They can then move freely in every direction, and if their movements are directed by an external force such as the electric current of the cathode, they are projected in one direction only like grapeshot from a cannon. On meeting an obstacle they produce by their molecular bombardment the effects of phosphorescence and heat, which the experiments of the illustrious physicists put in evidence.
This conception, now recognized to be inexact, was inspired by the old kinetic theory of gases which I will thus recapitulate. The molecules of gases are formed of perfectly elastic particles, a condition necessary to prevent their losing energy by impact, and are far enough apart from each other to exercise no mutual attraction. They are animated by a speed varying with the gas, calculated at about 1800 meters per second in the case of hydrogen, or about double that of a cannon-ball. This speed is also purely theoretical, for, by reason of their mutual impacts, the free path of each molecule is limited to about the thousandth part of a millimeter. It is the impact of these molecules which produces the pressure exercised by a gas on the walls that enclose it. If the space enclosing the same volume of molecules be reduced t one-half, the pressure is doubled. It is tripled when the space is reduced to one-third. It is this fact which is expressed by the law of Mariotte.
In a globe exhausted to a vacuum of the millionth of an atmosphere, things, according to Crookes, happen very differently. No doubt it still contains an enormous number of gaseous molecules, but the very great reduction in their number causes them to obstruct each other reciprocally much less than under ordinary pressure, and their free path is thus considerably augmented. If, under these conditions, a part of the molecules of air remaining in the tube be electrified and projected, as I said above, by an intense electric current, they may freely traverse space, and acquire an enormous speed, while at ordinary pressure, this speed is kept down by the molecules of air encountered.
The cathode rays, therefore, simply represented, in the original theory of Crookes, molecules of rarified gas, electrified by contact with the cathode, and launched into the empty space within the tube at a speed they could never attain if they were obstructed, as in gases at ordinary pressure, by the impact of other molecules. They were thought to remain, however, material molecules, not dissociated, but simply spread out, which would not change their structure. No one dreamed, in fact, at this epoch that the atome was capable of dissociation.
Nothing remains of Crookes’ theory since the measurement of the electric charge of the particles and of their mass has proved that they are a thousand times smaller than the atom of hydrogen, the smallest atom known. One might doubtless suppose in strictness, as was done at first, that the atom was simply subdivided into other atoms preserving the properties of the matter whence they came; but this hypothesis broke down in face of the fact that the most dissimilar gases contained in Crookes’ tubes gave identical products of dissociation, in which were fond none of the properties of the substances from which they had issued. It had then to be admitted that the atom was not divided, but was dissociated into elements endowed with entirely new properties which were identical in the case of all substances.
It was not, we shall see, by any means, in a day that the theory of dissociation just briefly indicated was established; in fact, it was clearly formulated only after the discovery of the radioactive substances and the experiments which helped me prove the universality of the dissociation of matter. And it was only after several years that physicists at last recognized, conformably with my assertions, the identity of the cathode rays with the effluves of particles emitted by ordinary substances during their dissociation.
(2) The Interpretations Now Current
At the time when only the cathode rays were known, the explanation by Crookes of their nature seemed to be quite different. On the discovery of the x-rays and of the emissions of the spontaneously radioactive bodies, such as uranium, the insufficiency of the old theory was made clear. One of the manifestations of the x-rays and of the radioactive emissions which made the greatest impression on the physicists and was the origin of the current explanations, was the production of electricity on all bodies both solid and gaseous struck by the new radiations. The x-rays and the emissions from radioactive bodies possess, in fact, the common characteristic of producing something which renders the air and other gases conductors of electricity. With these gases thus made conducting we can, by passing them between the plates of a condenser, neutralize electric charges. It was, as a consequence, admitted that they were electrified.
This was a very unforeseen phenomenon, for all earlier experiments had without exception shown that gases were not capable of being electrified. They can be kept, in fact, indefinitely in contact with a body electrified to a very high potential without absorbing any trace of electricity. If it were otherwise, no electrified surface — the ball of an electroscope, for instance — could retain its charge, and we were, therefore, in face of an entirely new fact, much more novel even than was at first thought, since it implied, in reality, the dissociation of matter, which nobody then suspected.
So soon as an unforeseen fact is stated, one always tries to connect it with an old theory, and since one theory alone, that of the ionization of saline solutions in electrolysis, gives an apparent explanation of the newly observed facts, haste was made to adopt it. It was therefore supposed that in a simple body there exits, as in a compound, two separable elements, the positive and negative ions, each charged with electricity of contrary sign. But the earlier theory of ionization only applied to compound bodies, and not to simple ones. The elements of compound bodies could be separated, as we now say, ionized — chloride of potassium, for instance, being capable of separation into its chlorine ions and its potassium ions; but what analogy could exist between this operation and the dissociation of chloride or potassium itself, since it was considered a fundamental dogma that a simple body could not be dissociated. There was all the less analogy between the ionization of saline solutions and that of simple bodies, that, when the elements of a salt are separated by the electric current, very different bodies are extracted according to the compound dissociated. Chloride of potassium, mentioned above, gives chlorine and potassium; with sodium oxide, oxygen and sodium are obtained, and so on. When, on the other hand, we ionize a simple body, we extract from it always the same elements. Whether it be hydrogen, oxygen, nitrogen, aluminum or any other substance, the substance extracted is the same, every time. Whatever may be the body ionized, and whatever the mode of ionization, one obtains only those particles — ions or electrons — of which the electric charge is the same in all bodies. The ionization of a saline solution and that of a simple body, such as a gas, for instance, are therefore two things which present, in reality, no analogy to each other.
From the verification of the fact that from simple bodies such as oxygen, hydrogen, etc., only the same elements can be extracted, it might easily have been deduced: first, that atoms can be dissociated; and secondly, that they are all formed of the same elements. These conclusions are now evident, but they were a great deal too much outside the ideas then dominant for any one to dream of formulating them.
The term ionization when applied to a simple body had no great meaning, but it formed the beginning of an explanation, for which reason it was eagerly accepted. I shall likewise accept it, in order not to confuse the reader’s mind, but at the same time shall take care to remark that the term ionization applied to a simple body merely means dissociation of its atoms, and not anything else.
Several physicists, it is true, and I am astonished to find Rutherford among them, think that the ionization of a gas can take place without in any way changing the structure of its atoms. One cannot see why that which is admitted to be exact in the case of a solid body should be otherwise for a gaseous one. We know that by divers means we can dissociate any simple body whatever. In the case of radium, aluminum, oxygen, or any other substance, the products of this dissociation are particles which are admitted to be exactly identical in the case of all bodies. There is therefore no foundation for saying that one has dissociated some substances and not others. To take something from an atom is always to begin its dissociation. Gases, on the other hand, are the easiest of all bodies to dissociate, because to accomplish this, it is only necessary to pass electric discharges through them.
This ionization of simple bodies — that is to say, the possibility of extracting from them positive and negative ions bearing electric charges of opposite signs — once admitted, presented a number of difficulties, which were studiously passed over in science, because it is really impossible to find their explanation. For these electric ions, or this ionic electricity, if I may use the expression, differs singularly in its properties from the ordinary electricity which a century of researches has made known to us. A few comparisons will suffice to show this. On any insulated body whatever we can fix only a very small quantity of electricity if it is a solid, and none at all if it is a gas. Ionic electricity, on the other hand, must necessarily be condensed in immense quantities on infinitely small particles. Ordinary electricity, even though it has the intensity of lightning, can never pass through a metallic plate connected with the earth, as Faraday showed long ago. On this classic property there has even been founded the manufacture of clothes from light metallic gauze which affords the workmen in factories, where electricity at a high potential is produced, protection from even the most violent discharges. Ionic electricity, on the other hand, easily traverses metallic enclosures. Ordinary electricity goes along wire conductors with the rapidity of light, but cannot be led like a gas into a hollow tube bent back upon itself. Ionic electricity, on the other hand, acts like a vapor, and can circulate slowly through a tube. And finally, ionic electricity has the property of giving birth to the x-rays whenever the ions animated by a certain speed happen to touch any body whatever.
No doubt it can be urged that electricity generated by the ionization of matter which has assumed the special form of electrical atoms, must possess in this form properties very different to ordinary electricity. But then, if the properties of the atom called electrical are absolutely different to electricity, why call it electrical. In the experiments I shall set forth, electricity will most often appear to us as an effect and not a cause. It is to this unknown cause what electricity is to the heat or the friction which generates it. When a rifle-ball or a jet of steam produces electricity by its impact, we do not say that this bullet or this jet of steam are electricity, nor even that they are charged with it. The idea would never enter any one’s head of confounding effect with cause as some persist in doing in the case of the radioactive emissions.
The phenomena observed in the dissociation of matter, such as the emission of particles having a speed of the order of light and the property of generating x-rays, are evidently characteristics possessed by none of the known forms of electricity, and ought to have led physicists to suppose, as I did, that they are certainly the consequence of an entirely new form of energy. But the imperious mental need of seeking for analogies, of comparing the unknown with the known, has led to the connecting of these phenomena with electricity under the pretext that among the effects observed one of the most constant was the final production of electricity.
It is plain, however, that several physicists are very near arriving by different roads at the conception that all these radioactive emissions which it is sought to connect with electricity by the theory of ionization, represent manifestations of intra-atomic energy — that is to say, of an energy which has no relation to anything known; and the facts proving that electricity is only one of the forms of this energy are multiplying daily.
One of the most important of these is the discovery due to Rutherford, of which I shall soon have to speak, namely, that the greatest part of the particles emitted during radioactivity proceed from an emanation possessing absolutely no electric charge, though capable of giving birth to bodies able to produce electricity. Emanation, ions, electrons, x-rays, electricity, etc., are really, as we shall see, only different phases of the dematerialization of matter — that is to say — of the transformation of intra-atomic energy.
“It seems”, wrote Prof. de Heen with regard to my experiments, “that we find ourselves confronted by conditions which remove themselves from mater by successive stages of cathode and x-ray emissions and approach the substance which has been designated the ether. The ulterior researches of Gustave Le Bon have fully justified his first assertions that all these effects depend upon a new mode of energy. This new force is as yet as little known as was electricity before Volta. We simply know that it exists”.
But whatever may be the interpretations given to the facts revealing the dissociation of matter, these facts are incontestable, and it is only the demonstration of them which is at present of importance.
On these acts there is almost complete agreement at the present time, and it is, the same with the identity of the products of the dissociation of matter, whatever be the cause of this dissociation. Whether they are generated by the cathode of Crookes’ tube, by the radiation of a metal under the action of light, or by the radiation of spontaneously radioactive bodies such as uranium, thorium, and radium, etc., the effluves are of the same nature. They are subject to the same magnetic deviation, the relation of their charge to their mass is the same. Their speed alone varies, but it is always immense.
We can, then, when we wish to study the dissociation of matter, choose the bodies in which the phenomenon manifests itself most intensely — either, for example, the Crookes’ tube, in which a metallic cathode is excited by the electric current of an induction coil, or, more simply, very radioactive bodies such as the salts of thorium or of radium. Any bodies whatever dissociated by light or otherwise give, besides, the same results, bit the dissociation being much weaker, the observation of the phenomena is more difficult.
Chapter II: The Products of the Dematerialization of Matter (Ions, Electrons, Cathode Rays, etc.)
(1) Classification of the Products of the Dematerialization of Matter
I have set forth in the preceding chapter the genesis of the current ideas on the interpretation of the facts relating to the dissociation of matter. We will now study the characteristics of the products of this dissociation. Not to complicate a subject already very obscure, I will accept, without discussion, the theories at present admitted, and will confine myself to the attempt to state them with more precision, and to bring together things which resemble one another, but which are often called by different names.
I have said that, whatever the body dissociated and the mode of dissociation employed, the products of this dissociation are always of the same nature. Whether it be the emissions of radium, of those of any metal under the influence of light, of those produced by chemical reaction or by combustion, or of those proceeding from an electrified point, etc., the products will, as already said, be identical, although their quantity and their speed of emission may be very different.
This generalization has taken a long time to establish. It was, consequently, natural that things recognized later on as similar after having first been considered as different, should have been designated by particular terms. It is therefore clearly important to define first of all the exact value of the various terms employed. Without exact definitions no generalization is possible. The necessity of such definitions makes itself all the more felt that the greatest confusion exists in the meaning of the terms generally in use. It is easy to see, moreover, why this should be so. A new science always gives birth to a new terminology. The science is not even constituted until its language has been fixed. The recently discovered phenomena necessarily compelled the formation of special expressions indicating both the facts and the theories inspired by those facts. But, these phenomena having been examined by various inquirers, the same words have sometimes received very different meanings.
Often words of old standing and possessing a well-defined meaning have been used to designate things newly discovered. Thus, for instance, the same word ion is used to designate the elements separated in a saline solution and those derived from the dissociation of simple bodies. Some physicists, like Lorentz, use indifferently the terms ions and electrons, which to others imply very distinct things. J.J. Thomson calls corpuscles [negative electrons] the electric atoms which Larmor and other authors call electrons, etc.
By only taking into account facts revealed by experiments and without troubling about the theories from which the definitions are derived, we find that the different products of the dissociation of matter now known may be arranged in the six following classes: (1) Emanations; (2) Negative Ions; (3) Positive Ions; (4) Electrons; (5) Cathode Rays; (6) X-rays and analogous radiations.
(3) Characteristics of the Elements Furnished by the Dissociation of Matter
The Emanation- This product, which we shall examine at greater length in the chapter devoted to the study of spontaneously radioactive matter, is a semi-material substance having some of the characteristics of a gas, but is capable of spontaneously disappearing into electric particles. It was discovered by Rutherford in thorium and by Dorn in radium, and according to the researched of J.J. Thomson (Cambridge Philos. Soc., April 1904, p. 391) it exists in the majority of ordinary bodies: water, sand, stone, clay, etc. It may, then, be considered as one of the usual stages of the dissociation of matter.
If we have just styled a semi-material substance “the emanation”, it is because it possesses at once the properties of material bodies and those of bodies which are not material or which have ceased to be so. It can be condensed, like a gas, at the temperature of liquid air, when, tanks to its phosphorescence, its behavior can be watched. It can be kept for some time in a sealed glass tube, but it soon escapes by transforming itself into electric particles and then ceases to be a material. These electric particles comprise positive ions (Rutherford’s rays), to which, after a certain time, succeed electrons (the same author’s beta rays) and x-rays (gamma rays). These various elements will be studied later on.
Although the “emanation” can produce electric particles by its dissociation, it is not charged with electricity.
Positive Ions and Negative Ions- Let us recall to mind, for the understanding of what is to follow, that, according to a theory already old, which has, however, taken a great extension in these days, all atoms contain electric particles of ascertained size, called electrons. Let us now suppose that a body of some kind, a gas for example, is dissociated — that is to say, ionized, as it is called. According to present ideas, there would be formed within it positive ions and negative ions by a process comprising the three following operations:
(1) The atom, originally neutral — that is to say, composed of elements which neutralize each other — loses some of its negative electrons. (2) These electrons surround themselves, by electrostatic attraction, with some of the neutral molecules of the gases around them in the same way that electrified bodies attract neighboring ones. This aggregate of electrons and neutral particles form the negative ion. (3) The atom, thus deprived of part of its electrons, then possesses an excess of positive charge, and in its turn surrounds itself with a retinue of neutral particles, thus forming the positive ion. Such is — reduced to its essential points — the present theory which the researches of numerous experimenters, especially J.J. Thomson, have succeeded in getting adopted, notwithstanding all objections raised against it.
Things, however, only happen in the manner described in a gas at ordinary pressure. In a vacuum, electrons do not surround themselves with a retinue of material molecules; they remain in the state of electrons and can acquire a great speed, so that the formation of negative ions is not observed in a vacuum. Nor does the positive ion in a vacuum surround itself with neutral particles, but, as it is composed of all that is left of the atom, it is still voluminous, which is why its speed is comparatively feeble.
It may happen, however, that this is the case with the emission from radioactive bodies, that the negative electrons are expelled from the atom into the atmosphere, at the ordinary pressure, with too great a speed for their attraction on the neutral molecule to be capable of exercise. They do not then transform themselves into ions, but remain as those emitted in vacuo. It is they that form the beta rays of Rutherford.
The positive ions, notwithstanding their volume, are likewise capable of acquiring a very high speed in the case of the emission from the radioactive substances. At least, such is the result of the researched of Rutherford, who supposes that the alpha rays — which constitute 99% of the emission of radium — are formed of positive ions launched with a speed equal to one-tenth that of light. This point demands elucidation by further researches.
When the factors of pressure and speed do not intervene, and the negative and positive ions are formed at atmospheric pressure, they have about the same bulk. It is only when they are generated in vacuo or are emitted with a very high speed that their dimensions vary considerably. In vacuo, in fact, the electron, as the nucleus of the negative ion, does not, as mentioned above, surround itself with material molecules, and remains in the state of electron. Its mass, according to several measurements of which I shall have to speak elsewhere, does not exceed the thousandth part of that of an atom of hydrogen. What remains of the atom deprived of a part of its electrons — that is to say, the positive ion — possesses a mass equal to and sometimes greater than that of an atom of hydrogen, and consequently at least a thousand times greater than that of the electron.
It is therefore necessary, when treating of the properties of ions, to distinguish — (1) whether they were formed in a gas at ordinary pressure; (2) if they were generated in vacuo; (3) if, by any cause whatever, they were launched into space at a great speed at the moment of their formation. Their properties vary according to these different cases, as we shall see in other parts of this work. But, in all these different cases, the general structure of the ions remains the same. Their fundamental nucleus is always formed of electrons — that is, of electric atoms.
It is natural to suppose that the dimensions and properties of the ions formed in a gas at ordinary pressure differ notably from those of the electrons, since these latter are supposed to be free from all admixture of matter. But it seems difficult, on the current theory, to explain some of the properties of the ions, especially those which can be observed with simple gases, bodies which are easy to ionize by many different means. It is noted that they then form in the aggregate an entirely special fluid of which the properties are akin to those of a gas, without, however, possessing its stability. It can circulate, for some time, before being destroyed, through a worm of metal connected with the earth, which electricity cold never do. It possesses a marked inertia, as its slight mobility proves. Such a fluid has properties too peculiar not to have a name given to it, for which reason I propose to call it the ionic fluid [plasma]. We shall see that, owing to its great inertia, we can transform it into very regular geometrical figures.
As ions are charged with electricity, they can be attracted by electrified bodies. This is, in fact, as we shall see later, the means of measuring their charges. When an ionized gas is enclosed between two metal plates, one of which bears a positive and the other a negative charge, the first-named attracts the negative and the last the positive ions. If the voltage of these plates is weak, part of the ions combine with one another, and become neutral, especially when their number is considerable. To extract them from the gaseous medium before they combine, it is necessary to raise the voltage of the containing vessel until the current produced by the circulation of the ions no longer increases — which maximum current is called the “saturation current”.
We shall likewise see, in the part of this work devoted to experiments, that if ions possess common properties, which allow them to be classed in the same family, they also possess certain properties which permit them to be sharply differentiated.
Electrons- The electrons, or electric atoms — called “corpuscles” by J.J. Thomson — are, as we have seen, the nucleus of the negative ion. They are obtained, discharged from any foreign element, by means either of Crookes’ tubes (when they take the name of cathode rays) or of radioactive bodies (when they are termed beta rays). But, in spite of these differences of origin, they appear to possess similar qualities.
One of the most striking properties of electrons — apart from that of generating x-rays — is that of passing through metallic plates without losing their electric charge, which, I repeat, is contrary to a fundamental property of electricity. The most violent discharges are, as is well known, incapable of passing through a metallic plate, however thin, connected with the earth.
These electrons, presumed to be atoms of pure electricity, have a definite size (and probably also a considerable rigidity). They have, whatever their origin, an identical electric charge, or can, at least, produce the same neutralization of an amount of electricity which is always the same. But we possess no means of studying them in repose; and they are only known to us by the effects they produce when animated by great speed.
Their apparent mass — that is to say, their inertia — is, as we shall see in another chapter, a function of their speed. It becomes very great, and even infinite, when this speed approaches that of light. Their real mass, if they have one in repose, would therefore be only a fraction of the mass they possess when in motion.
The measurements of the inertia of electrons have only been made with the negative electrons, the only ones which have yet been completely isolated from matter. They have not been effective with the positive ions. Being inseparable at present from matter, these last must possess its essential property — that is to say, a constant mass independent of speed.
Electrons in motion behave like an electric current, since they are deviated by a magnetic field, and their structure is much more complex, in reality, than the above summary would seem to indicate. Without going into details, I shall confine myself to saying that they are supposed to be constituted by vortices of ether analogous to gyroscopes. In repose, they are surrounded by rectilinear rays of lines of force. In motion, they surround themselves with other line of force — circular, not rectilinear — from which result their magnetic properties. If they are slowed down or stopped in their course they radiate Hertzian waves, light, etc. I shall recur to these properties in summing up in another chapter the current ideas on electricity.
The Cathode Rays- As has been said in a preceding chapter, physicists have greatly altered their views as to the nature of the cathode rays. They are now considered to be composed of electrons — that is to say, of atoms of pure electricity disengaged from all material elements. They are obtained by various processes, notably by means of radioactive substances. The simplest way to produce them in large quantities is to send an induction current through a glass bulb furnished with electrodes and exhausted to the millionth of an atmosphere. As soon as the coil begins to work, there issues from the cathode a sheaf of rays, termed cathodic, which can be deviated by a magnet.
The bombardment produced by these rays has as its consequence very energetic effects, such as the fusion of metals struck by it. From their actions on the diamond, the temperature they generate has been calculated at 3500 C. Their power of penetration is rather weak, whereas that of the x-rays, which are derived from them, is, on the contrary, very great. Lenard, who was the first to bring the cathode rays outside a Crookes’ tube, employed to close the orifice in the tube, a plate of aluminum only a few thousandths of a millimeter in thickness.
A portion of the electric particles constituting the cathode rays is charged with negative electricity; the other — that produced in the most central part of the tube — is composed of positive ions. These last have been called “Canal Rays”. The cathode rays and the canal rays of Crookes’ tubes are of the same composition as the alpha and beta radiations emitted by radioactive bodies such as radium and thorium.
Cathode rays possess the property of rendering air a conductor of electricity and of transforming themselves into x-rays so soon as they meet an obstacle. In the air they diffuse very speedily, differing in this from the x-rays, which have a strictly rectilinear progress. When Lenard brought the cathode rays out of a Crookes’ tube through a plate of thin metal, he noted that they formed a widely-spread fan which did not extend father than a few centimeters. In very rarified gases it is possible, on the other hand, by means of a diaphragm, to confine them to a cone free from diffusion for a length of a meter.
Whatever the gas introduced into a Crookes’ tube before creating the vacuum — a relative vacuum since there still remain in it thousands of millions of molecules, even when the pressure is reduced to the millionth of an atmosphere — it is noted that the cathode rays which are formed have the same properties and the same electric charges. J.J. Thomson has concluded from this that the atoms of the most different bodies contain the same elements. If, instead of a Crookes’ tube, a very radioactive matter, thorium or radium, is used, the majority of the proceeding phenomena are found with simply quantitative variations. For example, more rays charged with negative electricity are found in the Crookes’ tube than in those emanations of radium which are especially charged with positive electricity; but the nature of the phenomena observed in the two cases remains the same.
Speed and Charge of the Cathode and Radioactive Particles- The measurement of the speed and of the electric charge of the particles of which both bodies are found, has proved, as has just been said, the cathode rays and the emission from radioactive their identity. It would take long to set forth the divers methods which have settled these points. Details will be found in the memoirs of J.J. Thomson, Rutherford, Wilson, etc.. I will here indicate very briefly the principle of the methods used.
So far as the speed, which is of the same order as that of light, is concerned, it may seem very difficult to measure the velocity of bodies moving so quickly; yet it is very simple. A narrow pencil of cathodic radiations obtained by any means — for example, from a Crookes’ tube or a radioactive body — is directed onto a screen capable of phosphorescence, and on striking it a small luminous spot is produced. This sheaf of particles being electrified can be deviated by a magnetic field. It can therefore be deflected by means of a magnet so disposed that its lines of force are at right angles to the direction of the particles. The displacement of the luminous spot on the phosphorescent screen indicates the deviation which the particles undergo in a magnetic field of known intensity. As the force necessary to deviate to a given extent a projectile of known mass enables us to determine its speed, it will be conceived that it is possible to deduce from the extent of their deviation the velocity of the cathodic particles. It is seldom less than one-tenth that of light, or say 30,000 kilometers per second, and sometimes rises to nine-tenths. When the pencil of radiations contains particles of different speed, they trace a line more or less long on the phosphorescent screen instead of a simple point, and thus the speed of each can be calculated.
To ascertain the number, the mass, and the electric charge — or at least the ratio e/m of the charge to the mass — of the cathode particles, the procedure is as follows: The first thing is to ascertain the electric charge of an unknown number of particles contained in a known volume of gas. A given quantity of gas containing the radioactive particles is then enclosed between two parallel metallic plates, the one insulated and the other positively charged. The positive particles are repelled towards the insulated plate, while the negative particles are attracted, and their charge can be measured by the electrometer. From this total charge, the charge of each particle can evidently be deduced if the number of particles can be ascertained.
There are several modes of arriving at this number. The most simple, first used by J.J. Thomson, is based on the fact that when cathode particles are introduced into a reservoir containing water-vapor, each particle acts as a condensation nucleus for the vapor and forms a drop. The result is a cloud of small drops. These latter are far too small to be counted, but their number may be determined from the time they take to fall through the recipient containing them, the fall being very slow owing to the viscosity of the air. When one knows the number of these small drops, and consequently the number of cathode particles contained in a given volume of water vapor, and also the electric charge of all the particles, a simple sum in division gives the electric charge of each particle.
It is by working in this way that it has been possible to demonstrate that the electric charge of the cathode particles was constant whatever their origin (particles of radioactive bodies, of ordinary metals struck by light, etc.). Their electric charge is represented by about 10^8 electromagnetic units. The value e/m of the ion of hydrogen in the electrolysis of liquids being only equal to 10^5, it follows that the mass of the negative ion in dissociated bodies is the thousandth part of the atom of hydrogen, the smallest atom known.
The preceding figures only apply to negative ions. They are the only ones of which the size is constant for all substances. As to the positive ions which contain the greater part of the undissociated atom, their charge naturally varies according to the substance. Their dimensions are never less than those of the hydrogen atom.
The X-Rays- When the cathode rays — that is to say, the electrons emitted by a Crookes’ tube or by a radioactive body, meet an obstacle, they give birth to special radiations called x-rays when they come from a Crookes’ tube, and gamma rays when emitted by a radioactive body. These radiations travel in a straight line, and can pass through dense obstacles. They are not reflected, refracted, nor polarized, and this absolutely differentiates them from light. They are not deviated by a magnet, and this separates them sharply from the cathode rays, whose power of penetration is, besides, infinitely more feeble. The x- or gamma-rays possess the property of rendering air a conductor of electricity, and consequently of dissipating electric charges. They render phosphorescent various substances, and impress photographic plates.
When the x-rays strike any substance whatever, they cause the formation of what are called secondary rays, identical with the cathode rays; this simply means that x-rays derived from the dissociation of matter have the property of producing a further dissociation of matter when they come into contact with it, a property which luminous radiations, notably those of the ultraviolet region, likewise possess (1).
[(1) For further particulars of this analogy see C. Sagnac, L’Optique des Rayons X, p. 140, Paris 1900]
Notwithstanding the researches of hundreds of physicists ever since their discovery, our knowledge concerning x-rays is almost solely confined to the notice of the attributes described; and as they have no relation to anything known, they can be assimilated to nothing (2).
[(2) Prof. Soddy compares them to light, both being, according to him, pulses in the ether, and attributes the impossibility of their polarization, etc., to the fact that, unlike light, they are “sudden pulses very rapidly dying away” instead of regular successive undulations.]
It has been sought, however, to connect them with ultraviolet light, from which they would only differ by the extreme smallness of their wavelength. This hypothesis seems to have but small grounds for support. Without going into the speed which the cathode rays must possess to impart to the ether vibrations corresponding to those of light, and leaving on one side the absence of polarization and of refraction which would be justified by the smallness of the supposed waves, it is curious to observe that the more one advances into the ultraviolet region, and the nearer one consequently gets to the supposed wavelength of the x-rays, the less penetrating do the radiations become. In the extreme limit to the spectrum they end by being no longer able to overcome the slightest obstacle. For the extreme violet spectrum in the neighborhood of 0.160 to 0.1 microns, so lately studied by Schumann and Lenard, two centimeters of air are as opaque as lead, as is a sheet of mica the hundredth of a millimeter in thickness. Now, the x-rays, supposed to be so near to this extreme region of the ultraviolet, pass, on the contrary, through all obstacles, thick metal plates included. If they did not possess fluorescence and photographic action, no one would have dreamed of comparing them to ultraviolet light.
The impossibility of giving to the x-rays that deviation by a magnetic field which the cathode rays undergo, has caused them to be looked upon as no longer possessing any electricity, but this conclusion may easily be contested. Suppose, in fact, that the x-rays are constituted of electric atoms still more minute than the ordinary negative electrons, and that their speed of propagation borders on that of light. According to the researches to be presently mentioned, electrons having such a velocity would have an infinite mass. Their resistance to motion being infinite, it is evident that they could not be deviated by a magnetic field, though composed of electric elements.
What now seems to be most evident is that there is no more reason to connect the x-rays with electricity than with light. Assimilations such as these are the offspring of that habit of mind which induces us to connect new things with those previously known. The x-rays simply represent one of the manifestations of intra-atomic energy liberated by the dissociation of matter. They constitute one of the stages of the vanishing of matter, a form of energy having its own characteristics, which must be defined solely by these characteristics without endeavoring to fit it into previously arranged categories. The universe is full of unknown forces which, like the x-rays of today, and the electricity of a century ago, were discovered only when we possessed reagents capable of revealing them. Had phosphorescent bodies and photographic plates been unknown, the existence of x-rays could not have been verified. Physicists handled Crookes’ tubes, which yield these rays in abundance, for a quarter of a century without discovering them.
If it is probable that the x-rays have their seat in the ether, it seems certain that they are not constituted by vibrations similar to those of light. To me, they represent the extreme limit of material things, one of the last stages of the vanishing of matter before its return to the ether.
Having sufficiently described, according to present ideas, the supposed constitution of the products given off by matter during its dissociation, we will now study the various forms of this dissociation, and show that we shall everywhere meet again the elements just enumerated.
Chapter III: The Dematerialization of Very Radioactive Substances — Uranium, Thorium, Radium Etc.
(1) The Products of the Dematerialization of Very Radioactive Substances
We are about to relate, in this chapter, the researches which have been effected on very radioactive substances — that is to say, upon substances which dissociate spontaneously and rapidly. Among the products of their dematerialization we shall again meet with those which are given off by any substance dissociated by any means, but the products emitted will be much greater in quantity. Under different names we shall still find the emanation, ions, electrons, and x-rays.
It must not be thought that these substances represent all the stages of the dematerialization of matter. Those of which the existence is known are only parts of what is probably a very long series. If we always meet with the same elements in the products of all bodies subjected to dissociation, it is because the reagents actually in use, being only sensitive to certain substances, are naturally unable to reveal others. When we discover other reagents, we shall certainly note the existence of other elements.
The very great interest of the spontaneously radioactive substances consists in their emitting, ion considerable quantity, elements which other bodies only produce in much smaller quantity. By thus enlarging a general phenomenon, they permit of its being studied more in detail.
In this chapter we shall simply set forth the researches on eminently radioactive bodies, thorium and radium in particular. It is as yet a very new subject, and for that reason the results obtained will offer many contradictions and uncertainties. Their importance is, however, paramount.
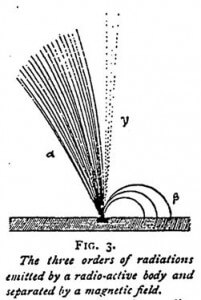
Rutherford, who has studied the radioactive substances with great success, and has, with Curie, discovered nearly all the facts concerning them, has designated their radiations by the letters alpha, beta, and gamma, which are now generally adopted. But under these new appellations are found exactly the products we have described. The alpha radiations are composed of positive ions, the beta radiation of electrons identical with those constituting the cathode rays, while the gamma radiations are similar to the x-rays. These three kinds of radiations are very clearly indicated in the diagram given in Figure 3.
To these several radiations is joined, as a primary phenomenon, according to Rutherford, the emission of a semi-material substance, which he terms “emanation”. It possesses no electric charge, but would appear to undergo subsequent stages of dissociation, which change it into alpha and beta particles. We will now examine the properties of the products we have just enumerated. For the most part, we shall only have to repeat or complete what has been said in a previous chapter.
(2) Alpha Rays, or Positive Ions
The alpha rays are formed of positive ions. They are deviated by an intense magnetic field, but in a contrary direction to the beta rays. The radius of curvature of their deviation is 1000 times greater than that of the beta particles. They form 99% of the total radioactivity of radium. They render air a conductor of electricity. Their action on a photographic plate is much less than that of the beta rays, and their force of penetration very slight, since they are stopped by a sheet of paper. This weak power of penetration enables them to be easily differentiated from the other radiations to which paper is no obstacle. Of all the emissions of radioactive bodies it is the alpha rays especially which make the air a conductor of electricity, and it is the beta rays which produce photographic impressions. When a radioactive body is enclosed in a glass tube nearly all the alpha particles are stopped by the glass walls.
It is supposed, from various calculations, that the alpha particles must have a mass equal or superior to that of the hydrogen atom and a like charge. Their speed, as calculated from the extent of their deviation by a magnetic field of given intensity, is one-tenth that of light. Their quantity varies according to the substance. For uranium and thorium it is, for one gram, 70,000 per second, and for radium a hundred thousand millions. This emission may last without interruption for more than a hundred years.
The emission of the alpha particles, otherwise positive ions, is, together with the production of the emanation, the fundamental phenomenon of radioactivity. The emission of beta particles and that of the gamma rays, which altogether form hardly one percent of the total emission, should represent a further stage in the dissociation of radioactive atoms.
On striking phosphorescent bodies the alpha particles render them luminous. It is on this property that is based the spinthariscope, an instrument which renders visible the permanent dissociation of matter. It simply consists of a screen of zinc sulfide, above which is placed a small metal rod, the end of which has been dipped in a solution of radium chloride. On examining the screen through a magnifying glass, there can be seen spurting out without cessation a shower of sparks produced by the impact of the alpha particles, and this emission may last for centuries, which shows the extreme smallness of the particles coming from the disaggregation of atoms. If this emission is visible, as Crookes says, because “each particle is made apparent solely through the enormous degree of lateral perturbation produced by its shock on the sensitive surface, in the same way that raindrops falling into the water produce ripples which exceed their diameter”. I have succeeded, by using certain varieties of phosphorescent sulfide, in making screens allowing the phenomenon of dissociation to be observed, not only with salts of radium, but also with divers substances, notably thorium and uranium (1).
[(1) The phosphorescent sulfide is spread in a layer, so thin as to be transparent, on a strip of glass first covered with varnish. The side coated with phosphorescent matter is then placed on the substance it is desired to examine, and the other face of the glass is observed through a magnifying glass. All uranium and thorium minerals, and even an ordinary incandescent mantle, give out a luminescent scintillation indicating a dissociation of matter; but in order to see this, it is necessary that the eye be rendered sensitive by previously remaining in the dark for a quarter of an hour.]
The high speed of the alpha particle seems very difficult to explain. This speed is intelligible enough in the case of the beta rays, which, being composed of atoms of pure electricity, and having, no doubt, a very small inertia, can acquire a very high speed under the influence of very minute forces; but for the alpha particles, whose dimensions would appear to be identical with that of the hydrogen atom, a velocity of 30,000 kilometers/second seems to be very difficult to explain, and I think that, on this point, the experiments of Rutherford and his pupils should be taken up anew (2).
[(2) it seems possible that this high speed can be explained by supposing that, although the alpha particles are being constantly emitted, it is only when they reach a certain velocity that their existence can be recognized by us. Thus, Strutt in reviewing Prof. Rutherford’s Radio-Activity (2nd ed.), says: “Ordinary matter may be emitting as many or more alpha particles than uranium, if only their velocity is less than that minimum velocity which has been found necessary to produce the characteristic phenomenon”. (Nature, 25 January 1906)]
It is hardly to be supposed, moreover, that these velocities are produced instantaneously; they are only comprehensible on the hypothesis that the particles of atoms can be compared to small planetary systems animated with enormous velocities. They would preserve their speed on leaving their orbits as does a stone launched from a sling. The invisible speed of rotation of the elements of the atom would therefore be simply transformed into a speed of projection visible or in any case perceptible by our instruments.
(3) The Beta Rays or Negative Electrons
Beta rays are considered to be composed of electrons identical with those of the cathode rays. They should, therefore, be formed of negative electric atoms, freed from all matter. Their mass should be, like that of the cathode particles, the thousandth part of that of the hydrogen atom. Their velocity should vary between 33% and 96% of that of light.
They are emitted in a much smaller proportion than that of the alpha particles, since they hardly form 1% of the total radiation. It is these rays which produce photographic impressions.
Their penetrating power is considerable. While the alpha rays are arrested by a sheet of ordinary paper, the beta rays will traverse several millimeters of aluminum. It is probably by reason of their great speed that they are much more penetrating than the cathode rays of a Crookes’ tube, which can only pass through sheets of aluminum of a thickness of some thousandths of a millimeter.
They immediately render luminous by impact bodies capable of phosphorescence, even when separated from them by a thin plate of aluminum. The phosphorescence is very bright in barium platinocyanide and those kind of diamonds — rather rare, by-the-by — which are capable of phosphorescence (1)
[(1) It is this very property which I have taken as a basis for the measurement of the intensity of the various samples of radium I have had occasion to examine. When the tube containing a salt of radium renders a diamond phosphorescent through a thin strip of aluminum, this salt may be regarded as very active. Brazilian diamonds alone — Cape diamonds never — are utilizable for this experiment. The first, in fact, are capable of phosphorescence by light and the second are not so. I have proved this by experiments extending to many hundreds of samples, details of which are given in my memoir on phosphorescence.
The beta particles seem to be somewhat complex, as is proved by the different speeds of their composing elements. This inequality of speed is easily recognized by the extent of the photographic impression they produce when submitted to the action of a magnetic field. It is likewise noticed, by covering the photographic plates with screens of varying thickness, that different alpha and beta particles possess different powers of penetration. It is therefore very probable that they represent well-marked stages of the dissociation of matter which we are not at present able to distinguish.
(4) The Gamma or X Rays
Together with the alpha and beta rays, the first charged with positive, and the second with negative electricity, radioactive bodies emit an extremely slight proportion (less than 1%) of gamma rays, entirely analogous, as to their properties, to the x-rays, but possessing a higher power of penetration, since they can traverse several centimeters of steel. This property enables them to be easily distinguished from the alpha and beta rays, which are stopped by a lead plate a few millimeters thick. Their nature is otherwise but little known, and if they are said to be analogous to the x rays, it is solely because they are not deviated by a magnetic field and possess great penetrating power.
What complicates to a singular degree the study of the above emissions (alpha, beta and gamma) is that none of them can touch a gaseous or a solid body without immediately causing — no doubt through the disturbance produced by their enormous velocity — a dissociation resulting in the production of rays called secondary, which are similar in their properties to the primary rays, but less intense. These secondary radiations also impress photographic plates, render the air a conductor of electricity, and are deviated by a magnetic field. They are able to produce, by their impact, tertiary rays having the same properties and so on. It is the secondary rays produced by the gamma rays which are the most active. A photographic impression through a metallic plate is sometimes intensified by the interposition of that plate, because the action of the secondary rays is then superposed on that of the primary rays.
(5) Semi-Material Emanation Proceeding from the Radioactive Substances
One of the most curious properties of the radioactive and, moreover, of all substances, is that of incessantly emitting a non-electrified product, designated by Rutherford as the emanation. This emanation represents the first stages of the dissociation of matter, and, by its disaggregation, generates emissions of the particles studied in the preceding paragraph. To this emanation is also due the property possessed by radium of rendering radioactive all bodies placed in its neighborhood.
The emanation has bee especially studied in the case of radium and thorium. Uranium does not give enough of it to be revealed by reagents. It ism however, very probable that, contrary to the opinion of Rutherford, it does disengage an emanation, since, according to the researches of J.J. Thomason, the majority of bodies in nature, water, sand, etc., produce one also.
The emanation can be drawn from any radioactive bodies, either by dissolving them in any liquid placed in a receiver communicating with a closed tube, or by bringing them to a red heat in a similar apparatus. The emanation drawn into the tube renders it phosphorescent by its presence, which fact allows of its behavior being examined. It can be condensed by the cold produced by liquid air. This condensation is revealed by the localization of the phosphorescence, but no substance capable of being measured by the balance appears. As the emanation of thorium condenses at 120° C, and that of radium at 150° C, it seems very likely that the emanations of different bodies, some resemblances notwithstanding, display various properties.
At the ordinary temperature radioactive bodies in a solid state emit the emanation, but only a hundredth part of the quantity emitted in the state of solution.
By introducing zinc sulfide into a bulb containing a solution of radium chloride, the disengagement of the emanation renders the sulfide phosphorescent. Radium, when heated, loses the greater part of its activity by reason of the quantity of emanation it gives off, but it regains it entirely in 20 days or so. The same loss occurs when a solution of this salt is heated to boiling.
When solid radium chloride has been brought to a red heat, or a solution of it has been boiled for some time, it still preserves a quarter of its primary activity, but this latter is then solely due to the alpha particles, as can be noted by the weak penetrating power of the rays emitted, which can no longer pass through a sheet of paper. It is only after a certain lapse of time that the appearance of the beta rays, capable of passing through metals, again takes place. The activity of the emanation os lost rather quickly. The rapidity of this loss varies according to the substance. That of actinium os destroyed in a few seconds, that of thorium in a few minutes, that of radium only at the end of three weeks, but it is already reduced by one-half in four days.
According to Rutherford, radium and thorium produce different kinds of emanation, that is, of dissociations which begin with the emission of the emanations. He has already counted five or six belonging to the last. The first engenders the second, and so on. They no doubt represent successive stages of the dematerialization of matter.
To the emanation are due three fourths of the heat incessantly produced by radium, which maintains its temperature at 3° or 4° C. above the ambient medium. If, in fact, radium be deprived of its emanation by heating, it gives out no more than a quarter of the heat it emitted at first. Almost all the rise in temperature is due to the alpha particles.
It results, as I have already remarked, from the experiments of Ramsay, that if some emanation of radium is left for some days in a tube, there can be observed the spectral lines of helium which were not there in the first instance.
Before drawing too many conclusions from this transformation, it must be remarked that helium is a gas which accompanies all radioactive minerals. It was even from these bodies that it was first obtained. This gas enters into no chemical combination (1), while it is the only substance hitherto found impossible to liquefy and can be kept for an indefinite time in the tubes in which it is enclosed.
[(1) Except cadmium]
This derivative of radium must be a very special helium since it appears to possess the property of spontaneously vanishing. Its sole resemblance to ordinary helium would seem to consist in the momentary presence of some spectral rays. It therefore seems very difficult to admit the transformation of radium into helium.
Rutherford considers the emanation as a material gas, because it can be diffused and condensed in the manner of gases. No doubt the emanation has some properties in common with material bodies, but dies it not curiously differ from these last by its property of vanishing in a few days, even when enclosed in a sealed tube, by transforming itself into electric particles? Here especially is shown the utility of the notion we have endeavored to establish, of an intermediary between the material and the immaterial — that is to say, between matter and the ether.
The emanation of the radioactive bodies represents, according to me, one of these intermediate substances. It is partly material, since it can be condensed and dissolved in certain acids and recovered by evaporation. But it is only incompletely material, since it ends by entirely disappearing and transforming itself into electric particles. This transformation, which takes place even in a sealed glass tube, has been proved by the experiments of Rutherford. He has shown that in disappearing the emanation at first gives birth to alpha particles and only later to beta particles and gamma radiation.
To prove that the emanation of radium or of thorium only generate at first positive or alpha particles, it is placed in a brass cylinder 0.05 mm thick, which retains all the alpha particles, but allows the beta particles and gamma rays to pass through. By noting at regular intervals by means of an electroscope the external radiation of the cylinder, it can be seen that it is only at the end of three or four hours that the beta particles appear. The alpha particles, on the contrary, show themselves at once, as is proved by their action on an electroscope connected with the interior of the cylinder.
Rutherford concludes form his experiments that “the emanation” at first emits only alpha rays, then beta and gamma rays by deposition the walls of the containing cylinder. It is difficult to conceive, from all we know of electricity, an emission of solely positive particles without a similar negative charge being produced at the same time.
However that may be, if the above theory be correct, the emanation in disappearing first produces positive ions relatively voluminous, then negative electrons, a thousand times less so, and finally gamma radiations.
Rutherford considers the emanation to be a sort of gas capable of spontaneously dissociating into electric particles expelled with immense velocity. In the course of dissociation this supposed gas would emit 3,000,000 times the amount of energy produced by the explosion of an equal amount of hydrogen and oxygen mixed in the proportions required for the formation of water. This last reaction is, however, as is well known, that which produces most heat.
Is this emanation, which produces so large a quantity of electrified particles, itself electrified? In no way. Rutherford asserts this positively, but this important point has been very clearly demonstrated by the researches of Prof. MacClelland. “The fact”, he says, “that the emanation is not charged has an important significance from the point of view of our conception of the manner in which the radium atom destroys itself. The radium atom assuredly produces alpha particles charged positively. But the particles of the emanation cannot be what remains of the atom after the emission of the alpha particles, for, in that case, they would be charged negatively”. There results from these experiments and the observations previously made by me that everything relating to the alpha particles, which form 99% of the emission of radioactive bodies, requires to be entirely re-examined.
(6) Induced Radioactivity
It is the emanation which, by freeing itself and by projecting its disaggregated particles on the surface of other bodies, produces the so-called induced radioactivity. This phenomenon consists in all substances placed in the neighborhood of a radioactive compound becoming momentarily radioactive. They do not become so if the active salt is enclosed in a glass tube. The beta and gamma rays are alone capable of producing induced radioactivity. The alpha particles do not seem to possess this power. Radioactivity, artificially provoked in any substance, disappears only after a fairly long time.
All glass or metals placed close to a radioactive substance or on which is blown, by means of a long tube, the emanation which it disengages, become momentarily radioactive. If it be admitted that this radioactivity is generated by the freeing of electric particles, it must be supposed that these particles are capable of being carried along by the air and of attaching themselves like dust to other bodies, and possess properties singularly different from those of ordinary electricity. Rutherford has verified the fact that the emanations of thorium can pass through water and sulfuric acid without losing their activity. If a metallic wire charged with negative electricity be exposed to the emanations of thorium, it becomes radioactive; if this wire be treated with sulfuric acid and the residuum then evaporated, it will be found that this latter is still radioactive. One really does not see how electricity could bear such treatment.
The induced radioactivity communicated to an inactive substance may be much more intense than that of the radioactive substance from which it emanates. When, in an enclosed vessel, containing some emanation from a radioactive body — thorium, for example, a metal plate charged with negative electricity at a high potential is introduced, all the particles emitted by the thorium concentrate themselves upon it, and, according to Rutherford, this plate becomes 10,000 times more active, surface for surface, than the thorium itself. These facts are not, any more than the preceding ones, explicable by the current theory.
If a metal, rendered artificially radioactive, be brought to a white heat, it loses its radioactivity, which spreads itself over the bodies in its neighborhood. Here again, we see the so-called electric atoms behave in a very strange manner.
The phenomenon of induced radioactivity is, then, quite inexplicable with the current ideas as to electric particles. It cannot be admitted that such particles deposited on a metal can be carried along by reagents. It would seems, from M. Curie’s experiments, that bismuth, plunged into a solution of radium bromide and carefully washed immediately, remains radioactive for at least three years. Can it be considered likely that electric particles act in such a manner? And, since they act so differently from electricity, how is it possible, as I have sp\o often repeated, to persist in applying to them the term “electric” atoms?
I must remark with respect to induced radioactivity that certain forms of energy can be stored in bodies for a great length of time and expend themselves very slowly. In my former experiments on phosphorescence I noted that calcium sulfide, exposed to the sun for a few seconds, radiates invisible light for 18 months, as is proved by the possibility of photographing the insolated object in the dark room or in the most complete darkness. At the end of 18 months it no longer gives any radiation, but still preserves a residual charge which persists for an indefinite period, and can be made visible by causing invisible infrared rays to fall on the surface of the insolated body.
A radioactive body has been compared to a magnet which keeps its magnetism forever, and can, without losing its power, magnetize other bodies. There is little foundation for this comparison, for the magnet is not the seat of a constant emission of particles into space (1). It might, however, be employed to explain roughly the phenomenon of induced radioactivity, which could be reduced to the fact that a radioactive body imparts its properties to a neighboring body, as the lodestone gives magnetism to fragments of iron near it. If the molecules of air were magnetic — and they are so in a slight degree, we should have a gas [radon], which, like the emanation of radioactive bodies, would be able to circulate in tubes and remain persistently on the surface of a metal without losing its properties.
[(1) M. Vallard’s experiments, however, have given him some reason to think that an electromagnet may, under certain conditions, actually emit particles of magnetism which he calls “magnetons”. See Revue Generale des Sciences, 15 May 1905.]
From all that has been set forth above one general consideration emerges, and this confirms what has been said at the commencement of this chapter — namely, that the stages of the dissociation of matter must be extremely numerous and that but few of them are yet known to us. Without being able to isolate them, we are at least certain that they exist. Since the unequal deviation of the beta particles by a magnet proves clearly that these are composed of different elements. We equally know that, in the semi-material product designated under the general name of emanation, already four or five very different stages of the dissociation of matter may be noted.
The same experiments equally confirm this other view — that mater, in dissociating, emits particles, more and more subtle, more and more dematerialized, which progressively lead to the ether. They themselves represent varied stages of dissociation, since their unequal deviation of the same magnetic field proves that they are composed of different elements. Finally, we come to the gamma radiations, which are no longer stayed by any obstacle, which no magnetic attraction can deviate, and which seem to constitute one of the last phases of the dissociation of matter before its final return to the ether.
Chapter IV: The Dematerializations of Matter — Methods Employed to Verify It
Many years have elapsed since I proved that the dissociation of matter observed in the substances called radioactive, such as uranium and radium, was, contrary to the ideas then accepted, a property belonging to all bodies in nature, and capable of manifesting itself under the influence of the most varied causes and even spontaneously. The spontaneous radioactivity of certain substances, such as uranium and thorium, which has so taken physicists by surprise, is in reality a universal phenomenon and a fundamental property of matter.
In a recent study (Proc. Cambridge Philos. Soc., April 1904, p. 391), Prof. J.J. Thomson has again taken up this question, and has succeeded in showing the existence of radioactivity in most bodies — water, sand, clay, brick, etc. He has drawn from them an “emanation” which is produced in a continuous manner, similar to that extracted by Rutherford from radium and having the same properties of radioactivity (1).
[(1) M. Blondlot, the well-known prof. from Nancy, on the other hand, has since made experiments that go to show that an emission capable of increasing the light of a phosphorescent screen, which can be activated by a magnetic or electric field or a draught of air, is emitted at ordinary temperatures by copper, silver, zinc, damped cardboard, all liquids, odorous substances such as camphor and musk, and the human body. See Comptes Rendu Acad. Sci. Paris 13 and 27 June, 4 and 25 July 1904.]
These experiments confirm all those I had already published on the spontaneous dissociation of matter, but they in no way prove, as Elster and Geitel would believe, that there is radium everywhere (1). It was the only explanation to which the last partisans of the indestructibility of matter could attach themselves. To admit that the atoms of two or three exceptional bodies can be dissociated is less embarrassing than to acknowledge that there is a question of an absolutely general phenomenon.
[(1) See also: Physikalische Zeitschrifte, 15 January 1906]
My experiments, moreover, take away all verisimilitude from such explanations. When we succeed in varying enormously the radioactivity of a body by certain chemical reactions, when we render greatly radioactive, by admixture, substances such as tin and mercury, which apart are not so, is it really possible to imagine that radium can have anything to do with the radioactivity then observed?
It was only thanks to long and minute experiments that I was able to establish the universality of the dissociation of matter. Some of these will be set forth in the second part of this work. Here only a summary of the results obtained will be given.
What phenomena now can be relied upon for the demonstration of the dissociation of the particularly radioactive substances, such as radium and thorium — that is to say, the production of particles emitted at an immense speed, capable of rendering the air a conductor of electricity and of being deviated by a magnetic field.
There exist other accessory characteristics: photographic impressions, production of phosphorescence and fluorescence, etc., by the emitted particles, but they are of secondary importance. Besides which, 99% of the emission of radium is composed of particles having no action on photographic plates, and there exist radioactive substances such as polonium which only emit rays such as these (1).
[(1) Since this was written, successful attempts have been made by Prof. Huff to impress a photographic plate with the beta rays from polonium: Proc. Roy. Soc., 21 July 1906 ]
The most important among the characteristics above enumerated is the emission of particles able to render the air a conductor of electricity and consequently capable of discharging an electroscope at a distance. It has been exclusively made use of in the separation of radium. It is therefore the one to which we shall principally have recourse.
The possibility of deviating these particles by a magnetic field constitutes the next most characteristic phenomenon. It has permitted the identity of the particles emitted by substances endowed with radioactivity, whether spontaneous or excited, with the cathode rays of Crookes’ tubes to be indisputably established. It is the degree of deviation of these particles by a magnetic field which has enabled their speed to be measured.
(2) Dissociation of Matter by Light
It was by attentively studying the action of light on metals and noting the analogy of the effluves emitted with the cathode rays that I was led to the discovery of the universality of the dissociation of matter.
It will be seen in the experimental part of this work that the technique of the experiments demonstrating the dissociation of bodies under the influence of light is pretty simple, since it amounts to throwing onto a positively charged electroscope the effluves of dissociated matter emitted by a metallic plate struck by light. These effluves are not produced by metals alone, but by the majority of substances. In some, the emission, surface for surface, may be 40 times more considerable than that produced by certain spontaneously radioactive substances, such as thorium and uranium.
For a long time the composition of these effluves which I asserted to be of the nature of cathode rays and of the radiations emitted by radioactive bodies was contested, but at the present day no physicist denies this identity.
The effluves produced under the action of light, like the cathode rays, render the air a conductor of electricity, and they are also deviated by a magnet. The electric charge of these component particles, as measured by J.J. Thomson, has been found equal to that of the cathode particles.
I shall show in the experimental part of this work that the different parts of the spectrum possess very different powers of dissociation, and that the resistance of various bodies to dissociation by light is very unequal. The ultraviolet is the most active region. In the extreme regions of the ultraviolet produced by electric sparks — regions which do not exist in the solar spectrum because they are absorbed by the atmosphere — it may be noted that all bodies dissociate with far greater rapidity than in ordinary light. In this part of the spectrum, substances which, like gold and steel, are not sensibly affected by solar light, emit effluves in quantities sufficiently abundant to discharge the electroscope almost instantaneously. If the earth were not protected from the extreme solar ultraviolet rays by its atmosphere, life on its surface, under existing circumstances, probably would be impossible.
Solar light does not possess the property of dissociating the molecules of gases. These can only be dissociated by the absolutely extreme ultraviolet radiations. If, as is probable, these radiations exist in the solar spectrum, before their absorption by the atmospheric envelope, an energetic dissociation of the aerial gases must take place on the confines of our air. This cause must have contributed, in the course of ages, to deprive certain stars, like the moon, of their atmosphere.
(3) Dissociation of Matter by Chemical Reactions
We now arrive at one of the most curious and unexpected parts of my researches. Convinced of the general character of the phenomena I had noted, I asked myself whether chemical reactions might not generate effluves similar to those produced from substances by light, and which would still possess the common characteristic of dissipating electric charges. Experiment has fully confirmed this hypothesis.
Here was a fact hitherto absolutely unsuspected. It had long been known, since the observation goes back as far as Laplace and Lavoisier, that hydrogen prepared by the action of iron on sulfuric acid was electrified. This fact ought to have impressed physicists the more that the direct electrification of a gas is impossible. A gas left for an indefinite period in contact with a metallic plate charged with electricity never becomes electrified. If the air could be electrified it would no longer be an insulator, an electroscope could no longer keep its charge, and the majority of electrical phenomena would still be unknown to us. But this fact, so important, since it contained the proof, then concealed, that matter is not indestructible, remained totally unnoticed.
The most striking phenomena hardly attract our attention except when light is thrown upon them by other phenomena, or when some great generalization capable of explaining them forces us to examine them more closely. If, in Lavoisier’s experiments just alluded to, hydrogen was found to be electrified, it was only because the atoms of this substance had undergone the commencement of dissociation. It is curious to note that the first experiment from which it could be deduced that matter is perishable had for its author the illustrious savant whose greatest claim to glory is that of endeavoring to prove that matter is indestructible.
The experiments collected at the end of this work prove that a large number of chemical reactions, whether accompanied or unaccompanied by the disengagement of gas, produce effluves similar to the cathode rays, and therefore reveal a destruction of matter without return during the reactions.
Among the reactions I shall only mention the decomposition of water by zinc and sulfuric acid or merely by sodium amalgam, the formation of acetylene by calcium carbide, the formation of oxygen by the decomposition of oxygenated water by means of manganese dioxide, and the hydration of quinine sulfate.
As regards quinine sulfate, it presents highly curious phenomena. This body, as it has long been known, becomes phosphorescent by the action of heat, but what was not known is that after having lost its phosphorescence, if sufficiently heated it becomes highly luminous and radioactive on refrigeration. After seeking the cause of its phosphorescence on cooling, and proving it to be due to a very slight hydration, I noted that by reason of this hydration the substance became radioactive for a few minutes. It was the first instance I discovered of the dissociation of matter — that is to say, of radioactivity — by chemical reactions, and it led me to the discovery of many more.
Since then, Dr Kalahne, Prof. of Physics at the University of Heidelberg, has taken up again the same subject in an important study. “My observations”, he says, “absolutely confirm that the chemical phenomena pointed out by Gustave Le Bon is the cause of the radiation” (Ann. Der Physik, 1905, p. 450, 457).
Rutherford also had my results relating to quinine sulfate verified by one of his pupils, who devoted a paper to the subject (1).
[Ms. Gates: Physical Review xviii, 1904, p. 144). She came to the conclusion that while Dr Le Bon is right as to the cause of the radiations, they differ from those of the radioactive substances in several particulars.]
The author has noted, as I did, that the air became a conductor of electricity, and that the phenomenon was duly produces, as I had said, by the hydration of quinine sulfate, but he thinks that the radioactivity is due to a chemical reaction to “to a kind of ultraviolet light” generated by the phosphorescence.
That the radioactivity was due to chemical reaction is exactly what I wished to demonstrate, and this Prof. Kalahne has confirmed; that it was due to ultraviolet light is impossible This Ms. Gates has since admitted in Physical Review 1906, p. 46), for the reason that the phosphorescence persists longer than the radioactivity, a thing which would not happen if the latter were the consequence of the light produced by the phosphorescence.
Rutherford thinks that the radiations thus produced differ from those of the radioactive substances because, he says, they have little penetrating power. He is not unaware, however, that this penetration proves nothing, since, according to him, 99% of the emission of radium is stopped by a thin sheet of paper, and certain very radioactive substances, such as polonium, only emit radiations having no penetration (cf. Prof. Giesel, Chem. Berichte 1906, Bd. xxxix, p. 780). I think that in writing the above the eminent physicist was still under the influence of the idea, very widespread at the outset, that radioactivity was the exclusive appanage of a small number of exceptional bodies.
(4) Dissociation of Matter by Electric Action
Certain very intense electric actions — for instance, induction sparks 50 cm long between which is placed the body to be experimented on — do exercise a slight action — that is to say, render the bodies submitted to their influence slightly radioactive, but the effect is much weaker than that produced by a simple ray of light or by heat.
This is not very astonishing. Electricity, as I shall show farther on, is a product of the dissociation of matter. It can certainly generate, like the cathode rays or radioactive emissions, secondary radiations in the substances struck by it, but the ions to which it gives birth in the air have too low a speed to produce much effect.
No doubt it is known, from the experiments of Elster and Geitel, that a wire electrified to a high potential acquires a temporary radioactivity; but it may be supposed in that case that the wire, by reason of its electrification, only attracts the ions which are always present in the atmosphere.
It was by pursuing the study of radioactivity excited by electricity that I was led to effect the experiment which will be mentioned later, and to compel particles of dissociated matter to traverse, visibly, and without deviation, thin plates of glass or ebonite.
(5) Dissociation of Matter by Combustion
If slight chemical reactions, such as simple hydration, can provoke the dissociation of matter, it will be conceived that the phenomena of combustion, which constitute powerful chemical reactions, must realize the maximum of dissociation. This is, in fact, what is observed. A burning body is an intense source of cathode rays similar to those emitted y a radioactive body, but possessing, by reason of their low speed, no great penetration.
For at least a century it has been known that the gases arising from flames discharge electrified bodies. Branly has shown that, even when cooled, gases preserve this property. All these facts remained uninterpreted, and it was hardly suspected that within them dwelt one of the proofs of the dissociation of matter.
This was, however, a conclusion to which one was bound to come. It has been clearly confirmed by the recent researches of J.J. Thomson. He has shown that a simple metal wire or thread of carbon brought to a white heat — the carbon thread of an incandescent lamp, for example — is a powerful and almost unlimited source of electrons and ions — that is to say, of particles identical with those of radioactive bodies. He has proved it by showing that the relation of their charge to their mass was the same. “We are therefore brought to this conclusion”, he says, “that from an incandescent metal or a heated thread of carbon electrons are projected”. Their quantity is enormous, he points out, for the quantity of electricity which these particles can neutralize corresponds to many amperes per square cm of surface. No radioactive body could produce electrons in such proportions. If it be considered that the solar spectrum indicates the presence of muych carbon in its photosphere, it follows that the sun must emit an enormous mass of electrons, which, on striking the upper layers of our atmosphere, perhaps produce the aurora borealis through their property of rendering rarified gases phosphorescent. This observation squares perfectly with my theory of the maintenance of the sun’s heat by the dissociation of the matter of which it is composed.
(6) Dissociation of Matter by Heat
Heat much inferior to that produced by combustion — that is to say, not exceeding 300° C. — is sufficient to provoke the dissociation of matter. But in this case the phenomenon is rather complicated, and its explanation has required very lengthy researches.
The reason is that, in reality, heat does not in this case appear to act directly as the agent of dissociation. I shall show in the chapter devoted to my experiments that it acts as if the metal contained a limited provision of a substance similar to the emanation of radioactive matter, which it gives out under the influence of heat, and then only recuperates by repose. It is for this reason that, after a metal has been rendered radioactive by a slight heat, it soon loses all trace of radioactivity, and regains it only after several days. It is, too, in this way that radioactive substances really behave, but in consequence of their activity being much superior to that of ordinary substances, whatever they lose from time to time is again formed simultaneously, unless they are brought to a red heat. In this last case the loss is only made up after a certain lapse of time.
When I published these experiments, J.J. Thomson had not yet made known his researches which proved that nearly all substances contain an emanation comparable with that of radioactive bodies, such as radium and thorium. His observations fully confirm my own.
(7) Spontaneous Dissociation of Matter
The experiments alluded to above prove that most substances contain a provision of radioactive matter which can be expelled by a slight heat and spontaneously formed anew; these substances are therefore, like ordinary radioactive substances, subject to spontaneous dissociation. It is, however, extremely slow.
In the foregoing experiments this spontaneous dissociation has only been made evident by means of slight heat. It is possible, however, by the help of various artifices — for instance, by folding the metal over itself so as to form a closed cylinder — to allow radioactive products to form therein, the presence of which is verified by the electroscope. The substance thus experimented on, however, soon ceases to be radioactive. It has not on that account used up all its provision of radioactivity; it has simply lost all that it can emit at the temperature under which the operation is effected. But, as with phosphorescent substances or radioactive matter, it suffices to heat it a little for it to produce an increased quantity of active effluves.
The researches I have just summarized prove that all substances in nature are radioactive, and that this radioactivity is in no way a property peculiar to a few bodies. All matter, then, tends spontaneously towards dissociation. This latter is most often very small, because it is hindered by the action of antagonistic forces. It is only exceptionally, and under different influences, such as light, combustion, chemical reaction, etc., capable of striving against these forces, that dissociation reaches a certain intensity.
Having proved by the experiments just summarized, of which the details will be found at the end of this volume, that the dissociation of matter is a general phenomenon, I am entitled to say that the doctrine of the invariability of the weight of atoms, on which all modern chemistry is based, is only an illusion resulting entirely from lack of sensitiveness in our balances. Were they sufficiently sensitive, all our chemical laws would be considered as merely approximation. With exact instruments we should note in many circumstances, and particularly in chemical reactions, that the atom loses a part of its weight. I may, then, be allowed to affirm that, contrary to the principle laid down as the basis of chemistry by Lavoisier, we do not recover in a chemical combination the total weight of the substances employed to bring about this combination.
(8) The Part Taken by the Dissociation of Matter in Natural Phenomena
We have just seen that very different causes acting in a continuous manner, such as light, can dissociate matter and finally transform it into elements which no longer possess any material properties, and cannot again become matter.
This dissociation, which has gone on since the beginning of the ages, must have played a great part in natural phenomena. It is probably the origin of atmospheric electricity, and no doubt that of the clouds, and consequently of the rainfall which exercises so great an influence on climate. One of the characteristic properties of radioactive emissions is that of condensing the vapor of water, a property which also belongs to all kinds of dust, and is demonstrated by an experiment of long standing (1). A globe full of water in ebullition is placed in communication with two other globes, one filled with ordinary air from a room, the other filled with the same air cleared of dust by simple filtration through cotton wool. It can then be seen that the stream coming into the globe containing the unfiltered air immediately condenses into a thick fog, while that in the globe containing pure air does not condense.
[(1) See Mr John Aitken: Trans. Roy. Soc. Edinburgh, vol. xxx (1883), p. 337; cf. C. Wilson, Philos. Trans. cxii, p. 403]
We see how the importance of the phenomenon of the dissociation of matter increases with the study of it. Its universality spreads daily, and the hour is not far distant, I believe, when it will be considered as the source of a great number of phenomena observed on the surface of our planet.
But these are not the most important of the phenomena due to the dissociation of matter. We have already shown it to be the source of solar heat, and we shall see presently that it is the origin of electricity.
Chapter V: Artificial Equilibria of the Elements Arising from the Dissociation of Matter
We shall see in a later chapter that the particles which escape from an electrified point connected with one of the poles of an electrical machine in motion are composed of ions and electrons of the same composition as the particles of dissociated matter emitted by the radioactive substances or by a Crookes’ tube. They, too, render the air a conductor of electricity, and are deviated by a magnetic field. If, therefore, we wish to study the equilibria of which the elements of dissociated matter are capable, we may replace a radioactive body by a point electrified by being connected with one of the poles of an electrical machine in action.
These particles are subject to the laws of attraction and repulsions which govern all electric phenomena. By utilizing these laws we can obtain at will the most varied equilibria.
Such equilibria can only be maintained for a moment. If we were able to isolate and fix them for good — that is to say, so that they would survive their generating cause — we should have succeeded in creating with immaterial particles something singularly resembling matter. The enormous quantity of energy condensed within the atom shows the impossibility of realizing such an experiment.
But if we cannot with immaterial things effect equilibria able to survive the cause which gave them birth, we can at least maintain them for a sufficiently long time to photograph them, and thus create a kind of momentary materialization.
By utilizing nothing but the laws mentioned above I have succeeded in grouping the particles of dissociated matter, so as to give this grouping every possible form — straight and curved lines, prisms, cells, etc., which were then made permanent by photography.
In Figures 8 to 11 we see straight and curved figures produced by the mutual repulsions of particles of dissociated matter having electrical charges of the same sign. So soon as the particles are brought near enough to each other, they repel one another and do not succeed in touching, as can be seen by the dark lines separating them and the considerable shortening of the radiation on the side where the particles are. By multiplying the discharges. By means of an arrangement of fine needles, the regular forms of Figures 12 to 15 are obtained.
The polygonal forms, represented in some of the photographs, are not, of course, reproductions of plane surfaces, but of forms really possessing three dimensions, of which photography can only give the projection. They are, therefore, really figures in space which I have obtained, by maintaining for a moment in the equilibrium forced upon them, particles of dissociated matter.
The particles which form the model of the images here produced, are not composed entirely of electrons. According to current ideas, they should be regarded as electric atoms surrounded by a retinue of material particles. They are therefore composed of those ions which we studied in a former chapter. But the nucleus of these latter is constituted of those electric atoms which are produced by the dematerialization of matter.
Among the forms of different equilibrium that we can cause particles of dissociated matter to assume, there is one — the globular form — of which the theory has not yet been established, attraction and repulsion not sufficing for its explanation. It is probable that the electric atoms must here be in a special state of whirling equilibrium. This equilibrium, though still momentary, is much more stable than those in the preceding experiments.
Electricity in this form has more than once been observed during storms, but rarely enough for its existence to have long been denied. In such cases, it occurs in the form of brilliant globes which may attain the size of a child’s head. They revolve slowly, and finally burst with a noise like a shell, causing great damage. The energy enclosed in them is therefore considerable, and I willingly appeal to this example for the comprehension of what may be done with condensed energy in a state of equilibrium of at least momentary stability.
We cannot hope to generate in our laboratories phenomena of such intensity, but we can reproduce them on a small scale. Small luminous spheres imitating globular thunderbolts can be produced by various methods. That of M. Stephane Leduc permits them to be very easily formed. It suffices to place on a photographic plate, at a few centimeters from each other, two very thin rods connected with the different poles of a static machine. There soon issues from the rod connected with the negative pole small luminous spheres, apparently about one mm in diameter, which very slowly make for the other rod, and vanish as soon as they touch it.
But, with this mode of operation, one may always suppose a particular form of effluve to exist between the two poles. I have therefore tried to obtain this globular electricity with a single pole., and I have succeeded in doing so by a very simple process. A rod, about half a cm in diameter, terminated by a needle of which the point is placed on a plate covered with silver bromide-gelatin, is connected with the negative pole of a Wimhurst machine, and the other pole is earthed. When the machine is set in motion, one sees issue from the point of the needle one or several luminous globes, which advance slowly and disappear abruptly after a few centimeters, leaving on the plate the trace of their trajectory.
If, instead of employing a thick rod terminated by a needle, a thin rod were used, the formation of luminous spheres would not take place. The phenomenon seems to act — though probably it is produced quite otherwise — as if the electricity of the thick rod accumulated at the point of the needle after the fashion of a drop of liquid [See: Kenneth Shoulders’ Elektrum Validum].
It is difficult to state precisely the part taken in these experiments by the gelatino-bromide of the photographic plate. Its presence facilitates the result, but is it indispensable? Some authors claim to have obtained globular electricity with simple plates of glass or mica, but I have not succeeded in producing them.
Howeve that may be, the luminous spheres formed by one of the processes just indicated, possess very singular properties, notably a considerable stability. They can be touched and displaced with a strip of metal without being discharged. A magnetic field — at all events the one of rather weak intensity at my disposal — has no action on them. If these spheres only consist of agglomerated ions, these last must be in a very special state. Their stability can only proceed from extremely rapid whirling movements, similar to those of the gyroscope which, as is well-known, simply owes its equilibrium to the rotary motion which animates it.
In the preceding experiments we have realized, with particles of dissociated matter, geometrical figures of a momentary stability which hardly survive the causes forming them. But it is possible to maintain for a fairly long time and on one surface certain forms of the electric fluid and to cause it to take the form of geometric plane figures with concise outlines.
In speaking of the properties of ionized gases, I have called by the name of ionic fluid, the fluid which the ionized particles make up by their aggregation. Thanks to its inertia, it is easy, by following the method pointed out by Prof. de Heen, to transform this into regular geometric figures possessing a certain permanence. The experiment is very simple. Take a large square plate of resin from 30 to 40 cm diameter and electrify it by passing its surface over one of the poles of an electrical machine in motion. Then expose the electrified face of this plate to two sources of ionization for several seconds — for instance, two Bunsen burners at a distance of 5 to 6 cm from each other. The ions starting from these sources come into contact with the plate, repel the electricity, and then, when face to face with each other, they halt and form a straight line (Figure 16), This invisible line is rendered visible by dusting powdered sulfur on the plate by means of a sieve. After slightly shaking the plate, there will only remain on its surface the straight line traced by the ionic fluid.
If, instead of two Bunsen burners, a certain number a re placed so as to form the outlines of geometrical figures, you obtain on the plate varied images: triangles, hexagons, etc., as regularly as if they had been traced with a ruler (Figures 17 to 19). It is evident that with an ordinary gas, you could produce nothing like this, since it would escape from the plate by diffusing through the atmosphere.
In the different experiments above mentioned, we have materialized, crystallized as it were, for an instant the fluid, so immaterial in appearance, composed of the union of the elements proceeding from the dissociation of matter. We now begin to see how, with more complicated equilibria and above all with the colossal forces she has at her command, Nature has been able to create those stable elements which constitute material atoms. While in evolution towards the state of matter, the ether must, no doubt, have passed through intermediate phases of equilibrium similar to those indicated in this chapter, and also through various forms the history of which is unknown to us.
Chapter VI: How, Notwithstanding Its Stability, Matter Can Dissociate
(1) Causes Capable of Modifying Molecular and Atomic Structures
The first objection which occurs to the mind of the chemist to whom one sets forth the theory of the dissociation of matter, is the following: How can bodies so stable as atoms — which appear to withstand the most violent reactions, since their weight is always recognized as invariable — dissociate either spontaneously or under such slight causes as rays of light hardly capable of influencing a thermometer?
To say, as I maintain, that matter is a large reservoir of forces, simply means that there is no need to look outside it for the origin of the energy expended during dissociation, but this in no way explains how intra-atomic energy condensed under an evidently very stable form can free itself from the bonds which hold it. The doctrine of intra-atomic energy therefore supplies no solution to the question just put. It is unable to say why the atom, which is to all appearance the most stable of all things in the universe, can, under certain conditions, lose its stability to the extent of easily disaggregating
If we wish to discover the solution of this problem, it will first be necessary to show, by various examples, that in order to produce in matter very great changes of equilibrium, it is not always the magnitude of the effort which counts, but rather the quality of that effort. Every equilibrium in Nature is only sensitive to the appropriate excitant, and it is this excitant which must be discovered in order to obtain the effect sought. Once discovered, it can be seen that very slight causes can easily modify the equilibrium of atoms and bring about, like a spark in a mass of gunpowder, effects whose intensity greatly exceeds that of the exciting cause.
A well-known acoustic analogy allows this difference between the intensity and the quality of the effort to be clearly shown from the point of view of the effects produced. The most violent thunderclap or the most deafening explosion may be powerless to cause the vibration of a tuning fork, while a sound, very slight but of suitable period, will suffice to set it in motion. When a tuning fork starts vibrating by reason of the production near it of a sound identical with its own, it is said to vibrate by resonance. The part played by resonance in acoustics as well as in optics is now well known; it gives the best explanation of the phenomena of opacity and transparency. It can help to explain, with all sthe facts I am about to state, that insignificant causes can cause great transformations in matter.
Although our means of observing the internal vibrations of bodies are very insufficient, facts, already numerous, prove that it is easy to profoundly change molecular and atomic equilibria, when they are acted upon by the proper agents. I shall confine myself to recalling a few of them.
A simple ray of light, though its energy is very slight, by falling on the surface of substances, such as selenium, silver sulfide, copper oxide, platinum black, etc., modifies their electrical resistance to a considerable extent. So, too, several dielectrics become birefringent when electrified. Boracite, again, which is birefringent at ordinary temperatures, becomes unirefringent when heated. Certain alloys of iron and nickel also become instantaneously magnetic by heat and lose their magnetism on cooling. Finally. If a transparent body placed in a magnetic body has a luminous ray passed through it, the rotation of the plane of polarization can be observed.
All these changes in physical properties necessarily imply changes of molecular equilibria. Slight causes suffice to bring about these changes because the molecular equilibria are sensitive to these causes. Forces far greater, but not appropriate, would, on the contrary, have no effect. Take any salt — potassium chloride, for instance. It can be ground, pulverized by the most powerful machinery without it ever being possible to separate the molecules of which it is composed. And yet, to dissociate these molecules (chlorine and potassium) it suffices to dissolve the substance in a liquid so that the solution is sufficiently diluted, according to modern theories on electrolysis.
Many similar examples can be given. To force apart the molecules of a steel bar it would have to be submitted to enormous mechanical strains; yet it suffices to heat it slightly, if only by placing the hand upon it, for it to elongate. This elongation of a bar by the contact of the hand can even be made visible, as Tyndall showed, to a whole audience by means of a lever and a mirror suitably arranged. A similar phenomenon is observed in water. It is almost incompressible under the very strongest pressure, and yet its temperature has only to be slightly lowered for it to contract.
We can produce in a metal far more through molecular displacements than those effected by heat, for there are some which imply a concrete change in the direction of the direction of the molecules. No mechanical force could cause such transformations; yet they are instantaneously effected by bringing a bar of iron near a magnet, when all its molecules instantaneously change their direction.
The recent employment of high temperatures, formerly impossible of attainment, as well as the introduction of the high electrical potentials which have permitted new chemical combinations to be produced, naturally leads us to think that it would be especially by means of these enormous forces that certain transformations will be possible. No doubt, by these new means, it has been possible to create certain chemical equilibria hitherto unknown, but to modify instable matter there is no need of these gigantic efforts. His is proved when we see certain luminous rays of a fixed wavelength producing instantaneously in various substances the chemical reactions which generate phosphorescence, and radiations of shorter wavelength giving birth to converse reactions which no less instantaneously destroy this phosphorescence. A further proof is afforded when we note that the Hertzian waves produced by electric sparks transform at a distance of 500 kilometers, the molecular structure of metal filings [in the coherer]; or again, when we observe that the neighborhood of a simple magnet immediately changes the direction of all the molecules of an iron bar in spite of all intervening obstacles.
In the dissociation of matter similar facts are observed. Metals that are highly radioactive under the influence of luminous radiations are hardly so under the radiations of one but slightly different. The same thing seems to occur here as in the phenomenon of resonance. It is possible, as I remarked above, to cause a tuning fork or even a heavy bell to vibrate by producing close to them a note of a certain vibratory period, when the most violent noises may leave them insensitive. When we become better acquainted with the causes capable of slightly dissociating the aggregate of energy condensed in the atom, we shall certainly arrive at a more complete dissociation and be able to utilize it for industrial purposes.
The whole of the preceding facts justifies my assertion that, in order to obtain important transformations of molecular equilibrium, it is not a question of the intensity but of the quality of the effort. These considerations enable it to be understood how structures so stable as atoms can be dissociated under the influence of such slight causes as a ray of light. If invisible ultraviolet radiations can dissociate the atoms of a steel block on which all the forces of mechanics would have no effect, it is because they form a stimulant to which matter is sensitive. The component parts of the retina are not sensitive to this stimulant, and this is why the ultraviolet light, capable of dissociating steel, has no action on the eye [except to blind it], which does not perceive its presence.
Matter, insensitive to actions of importance, can therefore be, I repeat, sensitive to very minute ones. Under the appropriate influences, a very stable body may become unstable. We shall see soon that sometimes imponderable traces of substances may at times powerfully modify the equilibria of other bodies and act in consequence as those excitants, light but appropriate, which matter obeys.
(2) Mechanism of the Dissociation of Matter
According to the ideas now current on the constitution of atoms, every atom may be considered as a small solar system comprising a central part round which turn with immense speed at least a thousand particles, and sometimes many more. These particles therefore possess a great kinetic energy. Let some appropriate cause come to disturb their trajectory or let their speed of rotation become sufficient for the centrifugal force which results from it to exceed the force of attraction that keeps them in their orbits, and the particles of the periphery will escape into space by following the tangent of the curve they formerly trod. By the emission they will give birth to the phenomena of radioactivity. Such, in any case, is one of the hypotheses which may be provisionally formulated.
When it was recognized that radioactivity was an exceptional property appertaining to only a very few bodies, such as uranium and radium, it was thought — and many physicists still think — that the instability of these bodies was a consequence of the magnitude of their atomic weight. This explanation vanishes before the fact shown by my researches that it is just those metals whose atomic weight is feeblest, such as magnesium and aluminum, which become most easily radioactive under the influence of light; while, on the contrary, it is bodies possessing a high atomic weight, like gold, platinum, and lead, which have weakest radioactivity. Radioactivity is therefore independent of atomic weight, and probably very often due, as I shall explain later on, to certain chemical reactions of an unknown nature. Two bodies not radioactive sometimes become so when combined. Mercury and tin may be placed among bodies of which the dissociation, under the action of light, is the weakest; I have shown, however, that mercury became extraordinarily radioactive under this same influence, so soon as traces of tin are added to it.
All the interpretations which precede contain assuredly only the outliners of an explanation. The mechanisms of the dissociation of matter is unknown to us. But what physical phenomenon is there whose ultimate causes are not equally hidden from our view?
(3) Causes Capable of Producing the Dissociation of Very Radioactive Substances
We have seen that various causes may produce the dissociation of ordinary matter. But in the dissociation of substances spontaneously very radioactive — radium and thorium, for instance — no internal cause seems to bring about the phenomenon. How, then, can it be explained?
Contrary to the opinions expressed at the commencement of researches into radioactivity, I have always maintained that the phenomena observed in radium arose from certain chemical reactions, similar to those produced in the case of phosphorescence. These reactions take place between substances of which one is in infinitesimal proportion to the other. I only published these considerations after I discovered bodies becoming radioactive in such conditions. Salts of quinine, for instance, are not radioactive. By letting them be slightly hydrated after desiccation, they become so, and remain phosphorescent while hydration lasts. Mercury and tin show no perceptible signs of radioactivity under the influence of light; but as to the former a trace of the latter, and its radioactivity at once becomes intense. These experiments even led me thereafter to modify entirely the properties of certain simple bodies by the addition of minute quantities of foreign bodies.
The disintegration of matter necessarily implies a change of equilibrium in the disposition of the elements which compose the atom. It is only by passing into other forms of equilibrium that it can lose part of its energy, and, in consequence, can radiate anything.
The changes of which it is then the seat differ from those known to chemistry, while the usual reactions affecting merely the structure of the groupings of atoms are extra-atomic. Ordinary chemistry can only vary the disposition of the stones destined to the building of an edifice. In the dissociation of atoms, the very materials with which the edifice is constructed are transformed.
The mechanism of this atomic disaggregation is unknown, but it is quite evident that it allows of conditions of a peculiar order, very different from those hitherto studied by chemistry. The quantities of matter put in play are infinitely small and the energies liberated extraordinarily large, which is the opposite of that which we get in our ordinary reactions.
Another characteristic of the intra-atomic reactions which produce radioactivity is that they seem to occur, as I said before, between bodies of which one is extremely small in quantity with regard to the other. These particular reactions, to which we will revert in another chapter, are mainly observed during phosphorescence. Pure bodies such as calcium sulfide, strontium sulfide, etc., are never phosphorescent. They only become so on being mixed with very small quantities of other bodies; and they then form mobile combinations, capable of being destroyed and regenerated with the greatest ease, which are accompanied by phosphorescence or the disappearance of phosphorescence. Other clearly defined reactions, such as a slight hydration, can likewise produce at the same time both phosphorescence and radioactivity.
This conception that radioactivity had its origin in a special chemical process, has at least secured the favor of several physicists. It has, notably, been adopted and defended by Rutherford and Soddy.
“Radioactivity”, say these, “is accompanied by a succession of chemical changes in which new types of radioactive matter are being continuously produced. It is a process of equilibrium when the amount of new radioactivity is balanced by the loss of the radioactivity already produced. Radioactivity is maintained by the continual production of new quantities of matter possessing temporary radioactivity” (Philos. Mag., Sept. 1902).
A radioactive body is, in fact, a body in course of transformation. Radioactivity is the expression of its never-ceasing leakage. Its change is necessarily an atomic disintegration. Atoms which have lost anything are, from that very fact, new atoms.
One might consider as singular — at all events, as little in accord with the observations in our laboratories — the existence of chemical reactions continuing almost indefinitely. But we also find in phosphorescence reactions capable of taking effect with extreme slowness. I have shown by my experiments on invisible luminescence that phosphorescent bodies are capable of retaining in the dark, and for two years after exposure to sunlight, the property of radiating, in a continuous manner, an invisible light capable of impressing photographic plates. Since chemical reactions can destroy phosphorescence, and continue to act for two years, it will be understood that other reactions, such as those capable of producing radioactivity, might last for very much longer.
Though the amount of energy radiated by atoms during their disaggregation is very large, the loss of material substance which occurs is extremely slight, by reason of the enormous condensation of energy contained in the atom. M. Becquerel estimates the duration of one gram of radium at a billion years. M. Curie contents himself with a million years. More modest still, Mr. Rutherford speaks only of a thousand years, and Sir William Crookes of a hundred years, for the dissociation of a gram of radium. These figures, of which the first are quite fantastic, become more and more reduced as the experiments become more exact. Dr Heydweiler (Physikalische Zeitscr., 15 Oct. 1903), after direct weighings, estimates the loss in 5 grams of radium at 0.02 mg in 24 hours. If the loss continued at the same rate, then 5 grams of radium would lose one gram of their weight in 137 years. We are already astonishingly far from the billion years imagined by M. Becquerel. Even Heydweiler’s figures, from certain of my experiments, are still too high. He has put in a tube the body experimented on in bulk, while I have noted that the radioactivity of a same body increases considerably if the substance is spread over a large surface, which can be obtained by leaving to dry the paper used to filter a solution ofit. We thus reach the conclusion that 5 grams of radium lose probably the fifth of their weight in 20 years and consequently that a gram would last 100 years, which are exactly the figures given by Sir William Crookes. In reality it is only repeated experiments which will finally settle this point.
But even if we accepted the figures of a thousand years given by Mr Rutherford for the duration of the existence of one gram of radium, it would be sufficient to prove that if spontaneously radioactive bodies, such as radium, existed in the geological epochs, they would have vanished long since, and would consequently no longer exist. And this again goes to support my theory, according to which rapid and spontaneous radioactivity only made its appearance since the bodies in question have been engaged in certain peculiar chemical combinations capable of affecting the stability of their atoms, which combinations we may perhaps some day succeed in reproducing.
(4) Can the Existence of Radium be Affirmed With Certainty?
If radioactivity be the consequence of certain chemical reactions, it would appear that an absolutely pure body cannot be radioactive. It was on this reasoning, supported by various experiments, that I based by assertion a few years ago that the existence of the metal radium was very problematic. In fact, although the operation of separating a metal from its combinations is very easy, it has never been possible to separate radium.
What one obtains at the present day under the name of radium is in nowise a metal, but a bromide or a chloride of this supposed metal. I consider it very probable that if radium exists and it is ever successfully isolated, it will have lost all the properties which render its combinations so interesting. But for a long time and for divers reasons I have predicted that radium will never be isolated, and, as the supposed process of isolation would be too simple not to have been tried by the possessors of sufficiently large quantities of radium, the complete silence observed upon these attempts is a strong presumption in favor of my hypothesis. The separation of barium from its salts is soeasy that this was one of the first metals isolated by Davy.
The preparation of the salts of radium enables us to guess the manner in which were possibly formed the unknown combinations which have given birth to radioactivity. One knows how salts of radium were discovered. M. Curie having noticed that certain uranium ores acted on the electroscope with more force than uranium itself, was naturally induced to endeavor to isolate the substance to which this special activity was due. The property registered by the electroscope of rendering air more or less a conductor of electricity being the only available means of investigation, it was the action on the electroscope which alone served as guide in these researches. It was through it alone, in fact, that one could ascertain in which part of the precipitates the most active substances were to be found. After dissolving the ore in various solvents and precipitating the products contained in these solvents by fitting reagents, the most active parts were, by means of the electroscope, set aside, redissolved and separated anew by precipitation, and these manipulations were repeated a great number of times. The operation terminated with fractional crystallization, and finally a small quantity of a very active salt was obtained. It is to the metal, not isolated yet, of the salt thus obtained that the name of radium was given.
The chemical properties of salts of radium are identical with those of the combinations of barium. Radioactivity apart, they only differ by certain rays in their spectra. The supposed atomic weight of radium, calculated from a very small quantity of its slats, varies so much with the different observers that nothing can be deduced from its as to the existence of this metal.
Without being able to pronounce positively, I repeat that I believe the existence of radium to be very disputable. It is, at any rate, certain that it has not been possible to isolate it. I should much more willingly admit the existence of an unknown compound of barium capable of giving this metal radioactive properties. Radioactive radium chloride seems to bear the same relation to inactive barium chloride that barium sulfide, impure but phosphorescent, bears to barium sulfide pure, and for that reason, non-phosphorescent. It suffices, as I have noted above, for traces of foreign bodies to be added to certain sulfides — those of calcium, barium, strontium, etc. — for them to acquire the marvelous property of becoming phosphorescent under the action of light. This phosphorescence which may be produced by radiation acting for no more than one-tenth of a second and destroyed, as I have shown, by other radiations of equally short period, proves the existence of chemical combinations of extreme mobility. Phosphorescence is a phenomenon which hardly astonishes us because it has so long been known; but on reflection, it must be acknowledged that it is quite as singular as radioactivity and still less explicable.
I will add that by operating with salts of radium but slightly active — that is to say, still mingled with foreign bodies — the role of the chemical reactions is very clearly apparent. Thus, for instance, the phosphorescence of these salts is lost by the action of heat and reappears after the lapse of a few days. Humidity destroys it altogether.
Whether, then, we take ordinary phosphorescence or radioactive properties, they both seem to be produced by chemical reactions the nature of which is totally unknown to us, but in which it seems one of the combining bodies is always in very small quantity compared to the other.
Doubtless, the law of definite proportions tells us that substances can only combine in certain relative quantities. This merely proves that bodies only form stable equilibria — which are the only ones accessible to chemistry — when combined in certain proportions. The number of combinations that two or more bodies can form is perhaps infinite, but as they are not stable, we can only suspect their existence when they are unaccompanied by marked physical phenomena. The combinations accompanied by radioactivity or phosphorescence are most probably instable combinations of this nature.
However this may be, the above theory greatly assisted me in my researches. It is owing to this theory that I was led to discover the radioactivity which accompanies certain chemical reactions, and to find combinations capable of enormously increasing the dissociation of a body under the influence of light, and, finally, to fundamentally modify the properties of certain simple substances.