Chapter I: The Constitution of Matter — The Forces Which Uphold Material edifices
(1) Former Ideas on the Structure of Atoms
Before setting forth the current ideas relating to the constitution of matter, I will briefly refer to those on which science has lived till now.
According to ideas which are still classical, matter is composed of small indivisible elements termed atoms. As these appear to persist in spite of all the transformations of bodies, it is supposed that they are indestructible. The molecules of bodies, the smallest particles subsisting which exhibit the properties of these bodies, are composed of a small number of atoms.
This fundamental notion has existed for over 2000 years. The great Roman poet Lucretius set it forth in the following terms, which modern books do little more than reproduce:
“Bodies are not annihilated when they disappear from our view. Nature forms new beings with their remains. It is only by the death of some that it grants life to others. The elements are unalterable and indestructible… The principles of matter, the elements of the great whole are solid and eternal: no foreign action can change them. The atom is the smallest body in nature… it represents the last term of division. There therefore exist in nature corpuscles of unchangeable essence” Their various combinations change the essence of bodies”.
Down to the last few years nothing had been added to the above except a few hypotheses on the structure of atoms. Newton regarded them as hard bodies incapable of deformation. Lord Kelvin supposed them to be constituted by vortices analogous to those which can be formed by striking the bottom of a rectangular box filled with smoke, the upper side of which is pierced with a hole. This causes vortices to issue in the frm of a ring composed of gaseous threads revolving round the meridians of the ring. The ring is displaced as a whole and is not destroyed by the contact of other rings. All these vortices offer permanent oscillations and vibrations, the intensity and frequency of which are modifiable by various influences such as that of heat.
It was largely on the old hypothesis of atoms that the theory termed atomic was founded during the last century. It was first supposed that all bodies brought to a gaseous state contain the same number of molecules in the same volume. Their weight, volume for volume, being supposed to be proportional to that of their atoms, it is possible, by simply weighing the body in a state of vapor, to ascertain what is called its molecular weight, from which is deduced, by a process of analysis that there is no need to show here, what is conveniently designated by the name of its atomic weight. It is compared with that of hydrogen taken as unity.
(2) Current Ideas on the Constitution of Matter
It is very difficult to set forth the current ideas on the constitution of matter, for they are still in the course of formation. We are in the midst of a period of anarchy, where we see the former theories vanishing and those springing up which will serve to build up the science of tomorrow.
The scholars who follow, in the reviews and scientific memoirs published abroad, the experiments and discussions to which are appended the names of the most eminent physicists, witness a curious spectacle. They see disappearing, day by day, fundamental conceptions of science which seemed established solidly enough to last forever. It is a regular revolution which is now in course of accomplishment.
The interpretations which flow from the facts recently discovered entirely upset the very bases of physics and chemistry, and seem destined to change all our conceptions of the universe. Our highest official teaching is, in France, too exclusively busy in seeing that the examination manuals are duly conned and is too hostile to general ideas toconcern itself about this prodigious movement. The new philosophy of the sciences now coming to light has no interest for it.
The scientific revolution now going on seems rapid, but this rapidity is much more apparent than real. The transformation of present ideas on the constitution of matter, which seemsto have taken only a few years, was prepared, in reality, by a century of researches.
Scientific ideas, in fact, only change with extreme slowness, and when they seem to be abruptly modified, it is always noted that this transformation is the consequence of a subterranean evolution which has taken long years to accomplish.
Five fundamental discoveries form the bases on which have been slowly built up the new ideas relating to the constitution of matter. They are: (1) the facts revealed by the study of electrolytic dissociation; (2) the discovery of the cathode rays; (3) that of the x-rays; (4) that of the bodies called radioactive, such as uranium and radium; (5) the demonstration that radioactivity does not belong exclusively to certain bodies and constitutes a general property of matter.
The oldest of these discoveries, since, in fact, it goes back to Davy, is that of the dissociation of chemical compounds by an electric current. Various physicists, notably Faraday, later completed its study. It has led in succession to the theory of atomic electricity and to the preponderating influence which the electric elements have in chemical reactions and the properties of bodies.
The second of the discoveries mentioned above give a glimmering idea that there might perhaps exist a condition of matter different to those already known; but this idea remained without any influence till Roentgen, examining more closely those Crookes’ tubes which physicists had been handling for 20 years without seeing anything in them, remarked that they gave out peculiar rays absolutely different to everything known, to which he gave the name x-rays. An unforeseen fact, absolutely new, and without any kind of analogy to known phenomena, thus burst into science.
The discovery of of the radioactivity of uranium and radium, and finally of the universal radioactivity of matter, very closely followed that of the x-rays. The link which connected all these phenomena, apparently so dissimilar, was not at first seen. It was established byb my researches that they formed but one thing.
Long before these last discoveries, it was well known that electricity played an important part in chemical reactions, but it was believed to be simply superposed on the material particles. By the discovery of electrolysis, Faraday had shown that the molecules of compound bodies carry a charge of neutral electricity of a definite and constant amount which is dissociated when solutions of metallic salts are traversed by an electric current. The molecules of bodies then came to be considered as composed of two elements, a material particle and an electric charge combined with it or superposed upon it.
The ideas most commonly accepted before the recent discoveries are well expressed in the following passage from a work published a few years ago by Dr Nernst, Prof.of Chemistry at the University of Gottingen:
“The ions are a kind of chemical combination between the elements or radicals and electric charges” the combination between matter and electricity is subject to the same laws as the combinations between different matters (laws of definite proportions, laws of multiple proportions)… If we suppose the electric fluid to be continuous, the laws of electrochemistry seem inexplicable; if, on the contrary, we suppose the quantity of electricity to be composed of particles of invariable size, the foregoing laws are evidently a consequence thereof. In the chemical theory of electricity, over and above the known elements there should be two others: the positive and the negative electrons”.
In this phase of the evolution of ideas, the positive electron and the negative electron were simply two new substances to be added to the list of simple bodies and capable of combining with them. The old idea of a material atom still persisted.
In the present period of evolution there is a tendency to go much farther. After asking themselves whether this material support of the electron was really necessary, several physicists have arrived at the conclusion that it is not so at all. They reject it entirely, and consider the atom to be solely constituted by an aggregate of electric particles without other elements. These particles can be dissociated into positive and negative ions, according to the mechanism explained above.
This was a gigantic step, and it is far from being one which all physicists have yet taken. A great uncertainty still dominates their ideas and their language. For the majority of them the material support remains necessary, and electric particles (electrons) are mingled with or superposed upon material atoms. These electrons, still according to them, circulate through conducting bodies, such as metals, with a velocity of the same order as that of light, by some mechanism totally unknown.
To the partisans of the exclusively electrical structure of matter the atom is composed solely of electric vortices. Round a small number of positive elements there are supposed to revolve negative electrons, not less than a thousand in number, and often more. Together they form the atom, which would thus be a kind of miniature solar system. “The atom of matter”, writes Larmor, “is composed of electrons, and nothing else” (Aether and matter, p. 137).
In its ordinary form the atom would be electrically neutral. It would become positive or negative only when freed from electrons of the contrary sign, as is done in electrolysis. All chemical actions would be due to the loss or gain of electrons. If, instead of being in a state of rapid motion, the electrons were in repose, they would precipitate themselves on each other, but the velocity by which they are animated causes their centrifugal force to balance their reciprocal attraction. When the speed of rotation is reduced from any cause whatever, such as a loss of kinetic energy due to the radiation of electrons into the ether, the attraction may gain the upper hand, and the electrons tend to unite; if it is, on the other hand, the centrifugal force which gains the day, they escape into space, as is verified in radioactive phenomena.
The atom, and consequently matter, is therefore in stable equilibrium, thanks only to the movements of the elements which compose it. These elements may be compared to a top, which fights against gravity as long as the kinetic energy due to its rotation exceeds a certain value. If it falls below this value, the top loses its equilibrium and falls to the ground. But the movements of atomic elements are far more complicated than those which have just been supposed. Not only are they dependent on one another, but they are also connected with the ether by their lines of force, and in reality only seem to be nuclei of condensation in the ether.
Such is, in broad outline, the current state of the ideas in course of formation as to the constitution of the atoms of which matter is formed. These ideas can very well be reconciled with those I have endeavored to establish in this work, according to which the atom is a colossal reservoir of energy condensed in the form already explained.
Whatever may be the future of these theories it may already be positively asserted that the ancient chemical atom, formerly considered so simple, is complicated in the extreme. It appears more and more as a sort of sidereal system having one or more suns and planets gravitating around it with immense velocity. From the structure of this system are derived the properties of the various atoms, but their fundamental elements seem to be identical.
(3) Magnitude of the Elements of Which Matter is Composed
The molecules of bodies, and a fortiori, the atoms, are extremely small. The most minute microbes are enormous colossi compared with the primitive elements of matter: yet various considerations have enabled their size to be estimated. They give figures which no longer appeal to the mind for the reason that infinitely small figures are as difficult to picture as infinitely large ones. But it is owing to the extreme smallness of the elements of which atoms are formed that matter in the course of dissociation can emit in permanent fashion and without appreciably losing weight, a veritable cloud of particles.
I have spoken in a former chapter of the millions of corpuscles per second which one gram of a radioactive body can emit for centuries. Such figures always provoke a certain amount of mistrust because we cannot succeed in representing to ourselves the extraordinary minuteness of the elements of matter. The mistrust disappears when one notes that very ordinary substances are capable, without undergoing any dissociation, of being for years the seat of an emission of abundant particles easily verified by the sense of smell, without this emission being discoverable by the most sensitive balances.
M. Berthelot has made on this subject some interesting researches (Comptes Rendu A.S.P., 21 May 1904). He has endeavored to determine the loss of weight undergone by very odoriferous though slightly volatile bodies. The sense of smell is infinitely superior in sensitiveness to that of the balance, since in the case of certain substances such as iodoform, the presence, according to M. Berthelot, of the hundredth of a millionth of a milligram can be easily revealed by it.
His researches have been made with this substance, and he has arrived at the conclusion that one gram of iodoform only loses the hundredth of a milligram in a year — one milligram in a century, though continuously emitting a flood of odoriferous particles in all directions. M. Berthelot adds, that if instead of iodoform, musk were used, the weight lost would be very much smaller, “a thousand times perhaps”, which would make 100,000 for the loss of one milligram. The same scholar also remarks, in a later work, “that there is hardly any metallic or other body which does not manifest, especially on friction, odors of its own, which is simply saying that all bodies slowly evaporate”.
These experiments give us an idea of the immensity of the number of particles which may be contained in an infinitesimal quantity of matter.
From various experiments, of which the most recent authors, Rutherford, Thomson, etc., have accepted the results, 1 cubic mm of hydrogen would contain 36,000 billions of molecules. These are figures the magnitude of which can only be understood by transforming them into units easy to interpret. An idea of their enormous magnitude will be obtained by finding out the dimensions of a reservoir capable of containing a similar number of cubic grains of sand having each a face or die of one mm. The above quantity of grains of sand could only be enclosed in a parallelepipedal reservoir with a base of 100 meters on each face and a height of 3,600 meters. These last figures would have to be much increases if we wished to represent the quantity of particles which one cubic mm of hydrogen would yield on the dissociation of its atoms.
(4) The Forces Which Maintain the Molecular Edifices
We have seen that matter is constituted by the union of very complicated structural elements termed molecules and atoms. We are compelled to suppose that these elements are not in contact; otherwise bodies could neither dilate, nor contract, nor change their state. We are likewise obliged to suppose that those particles are animated by permanent gyratory movements. The variation of these movements alone can explain, in fact, the absorption and the expenditure of energy which are noticed in the building up and the destruction of chemical compounds.
We ought, therefore, to picture to ourselves any body whatever, such as a block of steel or a rigid fragment of rock, as being composed of isolated elements in motion but never in contact. The atoms of which each molecule is formed themselves contain thousands of elements which describe round one or more centers, curves as regular as those of the celestial bodies.
What are the forces which keep together the particles of which matter is formed and prevent it from falling into dust? The existence of these forces is evident, but their nature remains totally unknown. The terms cohesion and affinity which are applied to them tell us nothing. Observation only reveals that the elements of matter exercise attraction nd repulsion. We can, however, add to this brief statement that the atom being an enormous reservoir of forces, it may be supposed, as I have already remarked in another chapter, that cohesion and affinity are manifestation of intra-atomic energy.
The stability of the molecular edifices bound together by cohesion is generally fairly great. It is, however, not enough to prevent chemistry from modifying or destroying it by various means, notably by heat. That is why it is possible to liquefy bodies, to reduce them to vapor, and to decompose them. The stability of the atomic vortices, of which the molecules are formed is, on the contrary, so great that it was deemed right to declare, after the experience of centuries, that the atom was unchangeable and indestructible.
The cohesion which keeps together the elements of bodies manifests itself by the mutual attraction and repulsion of the molecules; and the magnitude of the forces producing cohesion is measured by the effort we are compelled to make in order to change the form of a body. It resumes its primitive state when the action on it ceases, which fact proves the existence in the bosom of mater of forces of attraction. It resists the attempt to compress it, which demonstrates the existence of forces of repulsion when the molecules come within a certain distance of each other.
The attractions and repulsions by which cohesion is manifested are intense, but their radius of activity is extremely restricted. They cannot exercise any action at a distance, as does, for instance, gravitation. To nullify them we only require to separate the molecules of the body by heat. If the force of cohesion is abolished, the most rigid body is instantly transformed into liquid or vapor.
Outside the attractions and repulsions which operate between the particles of the same body, there are others produced between the particles of different bodies which vary according to their nature. We describe them under the general term of affinity; and it is they which determine the majority of chemical reactions.
The attractions and repulsions resulting from affinity engage the atoms in new combinations, or allow us to separate them from those combinations. Chemical reactions are only the destructions and restorations of equilibrium due to the affinities of the bodies present. One knows, by the effects of explosives, the power of the actions that affinity can produce when certain equilibria are disturbed.
It is from the manner in which the atoms are grouped by the energy of affinity that the molecular edifices result. They may be very unstable, and then the least stimulus, a shock or even the touch of a feather, suffice to destroy them. Such is the case with fulminate of mercury, iodide of nitrogen, and several other explosives. The edifice may, on the other hand, be so solid that it is destroyed with difficulty. Such are those organic salts of arsenic, like cacodylate of soda, wherein the molecule is so stable that no reagent can discover the quantity, enormous though it be, of atoms of arsenic which it contains. Aqua regia, fuming nitric acid, and chromic acid are without action on the molecular edifice; it is a strongly built fortress.
(5) The Attractions and Repulsions of Isolated Material Molecules and the Forms of Equilibrium Resulting from Them
The energies of affinity and cohesion are therefore manifested by attractions and repulsions. We have already seen that it is by these two forms of movement — whether in the case of material or of electric particles — that phenomena generally manifest themselves. This is why the study of them has always held a preponderating place in science; and many physicists still reduce the phenomena of the universe to the study of the attractions and repulsions of molecules subjected to the laws of mechanics. “All terrestrial phenomena”, said Laplace, “depend on molecular attractions, as celestial phenomena depend on universal gravitation”. Nowadays, however, it seems probable that the affairs of nature are more complicated. If attractions and repulsions appear to play so great a part, it is because of all the effects which forces can produce, these movements are the most easily accessible to us.
The equilibria determined by the attractions and repulsions which are born in the bosom of solid bodies, are discernible with difficulty, but we can render them visible by isolating their particles. The method is easy, since it only consists in dissolving the solids in some suitable liquid. The molecules are then nearly as free as if the body were transformed into gas, and it is easy to observe the effects of their mutual attractions and repulsions. It is well known, moreover, that the molecules of a dissolved body move within the solvent and develop there the same pressure as if they were converted into gas in the same space.
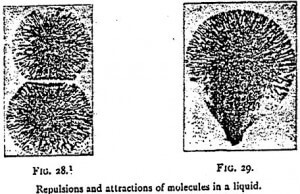
Such attractions exercised by molecules in a free state are of daily observation. To them are due the forms taken by a drop of liquid when it clings to the extremity of a glass rod. They are the origin of what has been called the surface tension of liquids, a tension in virtue of which a surface behaves as if it were composed of a stretched membrane. All attractions and repulsions can act only at a certain distance. As is known, the name of field of force is given to the space in which they are exercised, and that of lines of force to the directions in which are produced the attracting and repelling effects.
It is in the phenomena called osmotic that molecular attractions and repulsions are most clearly shown. When water is gently poured into an aqueous solution of a salt such as copper sulfate, we notice by the simple difference of color that the liquids are at first separate, but we soon see the molecules of the dissolved salt diffuse themselves through the supervening liquid. These consequently exists in them a force which enables them to overcome the force of gravity. This force of diffusion is the consequence of the reciprocal attraction of the particles of water and of the dissolved salt. It has received the name of osmotic pressure or tension.
All substances which possess the property of dissolving in a liquid attract the solvent, and conversely are attracted by it. Lime placed in a vessel rapidly attracts the vapor of water in the atmosphere, and increases in volume to the extent of breaking the vessel.
Osmotic attractions are very energetic. In the cells of plants they can make equilibrium to pressures of 160 atmospheres, and even more according to some authors. They are rarely less than 10 atmospheres.
Although the magnitude of osmotic pressure is considerable, 342 grams of sugar dissolved in a liter of water exercising a pressure of 22 atmospheres, this pressure does not manifest itself on the walls of the vessel, because the solvent opposes resistance to the movement of the molecules. To measure it, the substances present must be separated by a partition impermeable to one of them. Such partitions are called for this reason semi-permeable. It might be more correct, perhaps, to say unequally permeable. In the case of plant cells these partitions are formed by the walls of the cells.
In osmotic phenomena there are always produced two currents in a converse direction, called exosmose and endosmose, of which one may overcome the other. These simple molecular attractions and repulsions acting in the bosom of liquids govern a great number of vital phenomena, and are, perhaps, one of the most important causes of the formation of living beings. “Osmotic pressure”, says Van’t Hoff, “is a fundamental factor in the various vital functions of animals and vegetables. According to Vries, it is this which regulates the growth of plants; and, according to Massart, it governs the life of pathogenic germs”.
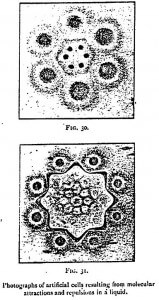
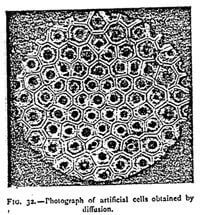
As the molecules existing in the midst of a liquid are able to attract or repel each other at a distance, they are necessarily surrounded by a field of force — a region in which their action is exercised. By utilizing the attractions and repulsions of the free molecules in a liquid, M. Leduc has succeeded in creating geometrical forms quite analogous to those of the cells of living beings. According to the mixtures employed, he has been able to bring before us particles which attract and repel each other, like electric atoms. By spreading over a glass a solution of potassium nitrate, on which are poured two drops of Indian ink 2 cm from each other, two poles are obtained whose lines of force repel each other. To obtain two poles of contrary sign, a crystal of potassium nitrate and a drop of defibrinated blood are placed at a distance of 2 cm from each other in a dilute solution of the salt mentioned above. By uniting several drops able to produce poles of the same sign, polyhedra are obtained with the appearance of the cells of living beings (Figure 32). If, finally, a salt be crystallized in a colloidal solution — gelatin, for instance — the field of force of crystallization being able to act in the contrary direction to the osmotic attractions, the form of the crystal becomes altered. These researches cast a strong light on the origin of the fundamental phenomena of life.
The above ideas on the constitution of matter may be summed up as follows: As soon as we lift the veil of appearances, matter, so inert in its outward aspect, is seen to possess an extremely complicated organization and an intense life. Its primary element, the atom, is a miniature solar system composed of particles revolving round one another without touching ad incessantly pursuing their eternal course under the influence of the forces which direct them. Were these forces to cease for a single minute, the world and all its inhabitants would instantly be reduced to an invisible dust.
On these prodigiously complicated equilibria of intra-atomic life are superposed, by reason of the association of atoms, other equilibria which complicate them further. Mysterious laws known solely by some of their effects, intervene to build with the atoms the material edifices of which the worlds are formed. Relatively very simple throughout the mineral kingdom, these edifices gradually become complicated, as we shall now show, and have finally, after the slow accumulations of ages, generated those extremely mobile chemical associations which constitute living beings.
Chapter II: Mobility and Sensibility of Matter — Variations of the Equilibria of Matter Under the Influence of the Surroundings
(1) Mobility and Sensibility of Matter
We have now arrived at that phase of the history of atoms where, under the influence of unknown causes of which we can only note the effects, the atoms have finally formed the different compounds which constitute our globe and the living beings upon it. Matter is born and will persist for a long succession of ages.
It persists with different characteristics of which the most distinctly apparent is the stability of its elements. They serve to construct the chemical edifices of which the form readily varies but of which the mass remains practically invariable throughout all changes. These chemical edifices formed by atomic combinations, appear to be firmly fixed, but are in reality of very great mobility. The least variations of the medium — temperature, pressure, etc. — instantaneously modify the movements of the component elements of matter.
The fact is, that a body as rigid in appearance as a block of steel, represents simply a state of equilibrium between its own internal energy and the external energies, heat, pressure, etc., which surround it. Matter yields to the influence of these last as an elastic thread obeys the pull exercised upon it, but regains its form — if the pull has not been too great — as soon as it ceases.
The mobility of the elements of matter is one of its most easily observed characteristics, since it suffices to bring the hand near the bulb of a thermometer to see the column of liquid immediately displaced. Its molecules consequently are separated by the influence of slight heat. When we place our hand near a block of metal, the movement of its molecules are likewise modified, but so slightly that it is not perceptible to our senses, and this is why matter appears to us to possess but little mobility.
The general belief in its stability seems to be confirmed, moreover, by observing that in order to subject a body to considerable modifications, to melt it or change it into vapor, for instance, very powerful means are required. Sufficiently exact methods of investigation show, on the contrary, that not only is matter of an extreme mobility, but is further endowed with an unconscious sensibility which cannot be approached by the conscious sensibility of any living being.
It is known that physiologists measure the sensibility of a being by the degree of excitement necessary to produce in it a reaction. It is considered very sensitive when it reacts under very slight excitants. Applying to mere matter a similar means of procedure, we note that the substance most rigid and least sensitive in appearance is, on the contrary, o an unexpected sensibility. The matter of the bolometer, reduced by final analysis to a thin platinum wire, is so sensitive that it reacts — by a variation of electric conductivity — when struck by a ray of light of such feeble intensity as to produce a rise in temperature of only the hundred millionth of a degree.
With recent progress in the means of examination this extreme sensitiveness of nature becomes more and more manifest. Mr. H. Steele has found that it is sufficient to touch an iron wire slightly with the finger for it to become immediately the seat of an electric current. It is known that hundreds of miles away the Hertzian waves greatly modify the state of metals with which they come in contact, since they change in enormous proportion their electric conductivity. It is on this phenomenon that wireless telegraphy is based.
The extraordinary sensibility of matter which has enabled the bolometer to be created and wireless telegraphy to be discovered, is utilized in other instruments employed in industry; such as, for instance, the telegraphone of Poulsen, which enables spoken words to be preserved and reproduced by the changes of magnetism brought about in the surface of a steel band moving between the poles of an electromagnet to which a microphone is attached. When you speak into the membrane of this last, the minute fluctuations of the current in the microphonic circuit cause variations of magnetism in the molecules of the steel ribbon of which the metal retains the trace. These variations permit us to reproduce the speech at will by passing the same band between the poles of an electromagnet put in circuit with a telephone.
This sensibility of matter, so contrary to what popular observation seems to indicate, is becoming more and more familiar to physicists. This is why such an expression as “the life of matter”, utterly meaningless 25 years ago, has come into common use. The study of mere matter yields ever-increasing proofs that it has properties which were formerly deemed the exclusive appanage of living beings. By taking as a basis this fact, “that the most general and most delicate sign of life is the electric response”, Mr Bose has proved that this electric response “considered generally as the effect of an unknown vital force” exists in matter. And he shows by ingenious experiments —the “fatigue” of metals and its disappearance after rest, and the action on these same metals of excitants, of depressants, and of poisons.
We must not be too much astonished at finding in matter properties which once seemed to belong solely to living beings, and it would be useless to seek therein a too simple explanation of the still impenetrable mystery of life. The analogies discovered are, it is likely, due to the fact that nature does not greatly vary her procedure and constructs all beings, from mineral to man, with similar materials, whence they are endowed with common properties. It always applies the fundamental principle of least action, which would suffice by itself to establish the fundamental questions of mechanics. It consists, as we know, in the enunciation, so simple and of such deep import, that of all roads which lead from one situation to another, a material molecule under the influence of a force can take but one direction, namely, the one which demands the least effort. It will probably be seen one day that this principle is not only applicable to mechanics but also to biology. It is perhaps also the secret cause of the laws of continuity observed in many phenomena.
(2) Variations of the Equilibria of Matter Under the Influence of the Medium
Matter is, then, like all beings, strictly dependent on the medium in which it finds itself, and is modified by the slightest changes in this medium. So long as these changes do not exceed certain limits, the velocity and amplitude of the movements of the material molecules are modified without any change in their relative position. If these limits are exceeded, the equilibria of matter are destroyed or transformed. The majority of chemical reactions show us such transformations.
But in every way matter is so mobile and so sensitive that the most insignificant changes in the medium — for instance, a rise or fall in temperature of a millionth of a degree — produce modifications which our instruments allow us to note.
Matter as we know it only represents, as I have said before, a state of equilibrium, a relation between the internal forces it contains and the external forces which act upon them. The last cannot be modified without a similar change in the first, as one pan of a balance cannot be touched without causing the other to oscillate. It may therefore be said, in mathematical language, that the properties of matter are a function of several variable factors, especially temperature and pressure.
These various influences are capable of acting separately, but they may also act in combination. Thus there exists a temperature, variable for each body, called critical, above which no body can exist in a liquid state. It then immediately becomes gaseous and remains so whatever pressure may be brought to bear on it. If water is heated in a closed tube, a time arrives when, suddenly, it transforms itself entirely into a gas so invisible that the tube seems totally empty. For a long time many gases could not be liquefied, precisely because it was not known that the action of pressure was null if the gas had not first been lowered below its critical point. Carbonic acid is very easily liquefied by pressure at a temperature below 31° C. Above that temperature no pressure can bring it to a liquid state.
Matter must therefore be considered as a most mobile thing, very unstable in equilibrium, and impossible to be conceived of apart from its surroundings. It possesses no independent property beyond its inertia, from which it derives the constancy of its mass. This property is absolutely the only one which no change of surroundings, pressure, temperature, etc., can alter. Take away from matter its inertia, and one does not see how it is possible to define so changeable a thing.
Notwithstanding the extreme mobility of matter, the world, however, seems very stable. It so so, in fact, but simply because, in its present state of evolution, the medium in which it is wrapped varies within rather narrow limits. The apparent constancy of the properties of matter results solely from the present constancy of the medium in which it is plunged.
This notion of the influence of the medium, rather neglected by the old chemists, has finally acquired great importance, since it has been proved that many reactions depend upon it, and vary in very different directions, according to the alterations, sometimes very slight, of temperature or of pressure. When the differences are considerable, many reactions are found to be entirely transformed, or to become impossible. If one could only examine substances at certain temperatures, one would consider them very different from the same substances observed at ordinary temperatures. At the temperature of liquid air, phosphorus loses its violent affinity for oxygen, and is without action upon it; sulfuric acid, which generally acts so markedly on litmus paper, no longer turns it red. At a high temperature we see, on the other hand, new affinities non-existent at ordinary temperatures come to light. Nitrogen and carbon, which combine with no other bodies at a low temperature, easily combine with several at 3000°, and form bodies hitherto unknown — calcium carbide, for example. Oxygen, which generally has no action on the diamond, acquires so energetic an affinity for this body at a high temperature that it combines with it and becomes incandescent. Magnesium has a rather mild affinity for oxygen, but at a sufficiently high temperature its affinity for it reaches such a point that, when plunged into an atmosphere of carbonic acid, it decomposes it, seizes upon its oxygen and burns continuously when lighted.
Thus, then, the elements of matter are in incessant motion: a block of lead, a rock, a chain of mountains have but an apparent immobility. They are subject to all the variations of the medium and are constantly modifying their equilibria to correspond to it. Nature knows no rest. If repose exists anywhere, it is neither in the world we inhabit nor in the beings on its surface; nor is it even existent in death, which only substitutes for certain momentary equilibria of atoms other equilibria whose duration will be as ephemeral.
Chapter III: The Various Aspects of Matter — Gaseous, Liquid, Solid, and Crystalline States
1) The Gaseous, Liquid, and Solid States
According to the external forces to which it is subjected, matter assumes three states, which have been called the solid, liquid, and gaseous. Yet the most recent researches have clearly proven that there exists no wide separation between them. The continuity of the liquid and gaseous states has been put in evidence by the researches of Van der Waals, and the continuity of the liquid and solid states by other experimenters. Under sufficient pressure, solids behave like liquids, their molecules slide one over the other, and a solid metal at length flows like a liquid. “The laws of hydrostatics and hydrodynamics”, says Spring, “are applicable to solids subjected to strong pressures”. This property of the hardest bodies of behaving like liquids under certain pressures has been utilized commercially in America for the manufacture of tools from blocks of steel subjected to sufficient pressure without the need of raising the temperature. Yet this metal may be regarded as the type of substances hardly malleable.
The crystalline state itself cannot establish a very clear separation between the solid and liquid states. These exist, as Lehmann has shown, semi-liquid crystals, and I myself have found a means of preparing crystals of a pasty consistency (simply by holding a strip of magnesium with a long pair of tongs for some minutes in mercury). We have seen above that liquids, while remaining liquids, can assume geometrical forms akin to the crystalline state, and certain optical processes allow us to reveal their existence.
In a general way, however, the crystalline state constitutes, as we shall see, a very peculiar stage of matter which gives it an individuality of its own, and approaches, from some points of view, that of living beings.
(2) The Crystalline State of Matter — Life of Crystals
Among the unknown forces of which we only perceive the existence by a few of their effects, are found those which compel the molecules of bodies to take strictly geometrical forms bearing the name of crystals. All solid bodies have a tendency towards the crystalline form (1). The geometrical equilibria from which these forms result, give a kind of individuality to the molecules of matter. Matter individualizes tehm in the same sense that the living being does — by incorporating the elements borrowed from the medium itself.
[(1) Prof. Quincke of Heidelberg has lately shown that all substances, on passing from the liquid to the solid state, assume what he calls a “foam structure”, or become a network of cells which may enclose crystals (Proc. Roy. Soc., 21 July `1906)
There is nothing out of the way in this expression — the individualization of matter — when applied to its transformation into geometrical bodies. The mineral being is characterized by its crystalline form as the living being is characterized by it anatomical one. They crystal also undergoes, like the animal or the plant, a progressive evolution before attaining its final form. Again, like the animal or the plant, the mutilated crystal can repair its mutilation. The crystal is in reality the final stage of a particular form of life.
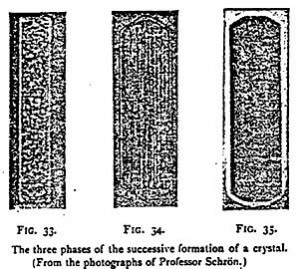
Among the facts which may serve as supports to these considerations, must be especially quoted the beautiful experiments of Prof. Schron on the successive transformations which cause material molecules to assume the crystalline form. The three principal ones are (10 a granular phase; (2) a fibrous phase; (3) a homogenous phase. They are represented by the three photographs here reproduced, which I owe to the courtesy of the scolar in question. In a solution about to crystallize are first formed globules, in the heart of which granulations soon appear (Figure 33). These granulations elongate and take a fibrous aspect (Figure 34), to which later on succeeds the homogeneous state (Figure 35), which constitutes the definitive form of the crystal. The crystal being has then terminated its cycle.
These laws of the formation of crystals are general, and can be observed in the crystals of mineral substances as well as in those which, according to Scron, accompany microorganism. Among the secretions of every microbe there always appear, according to him, crystal characteristic of every species of microbe.
These observations show tha during its pre-crystalline period — its infancy — the future crystal behaves like a living being. It represents tissue in the course of evolution. It is an organized being undergoing a series of transformation of which the final stage is the crystalline form, as the oak is the final stage of the evolution of the acorn. The crystal would therefore seem to be the last phase of certain equilibria of matter unable to rise to the forms of higher life.
Researches carried out in different directions confirm the above conclusions. Thus M. Cartaud has found that metals, polished and then attacked by picric acid dissolved in acetone, exhibit “a completely closed and microscopic network of cells… Cells and crystal show an evident affiliation. Pebbles with the same crystalline orientations have the characteristics of possessing a cellular web of specific form and disposition, which permits a crystal to be regarded as an aggregate of similar cells arranged in the same way”. Cellular structure would therefore seem to be an embryonic phase, and crystalline structure an adult phase.
Far from being an exceptional state, the crystalline form is, in reality, the one to which all forms tend, and which they attain so soon as certain conditions of the medium are realized. Salts dissolved in an evaporating solution, and a melted metal when cooled, always tend to assume the crystalline form; and if we consider, as we do nowadays, tha solutions show close analogies with gases, it may be said that the two most usual forms of nature are the gaseous and the crystalline.
There is hardly anything in nature but the crystal which possesses a truly stable and definite form. An ordinary living being is, on the other hand, something extremely mobile, always changing, and only continuing to live on the condition that it dies and is reborn unceasingly. Its form only appears definite because our senses can only perceive fragments of things. The eye is not made to see everything. It picks out of the ocean of forms that which is accessible to it, and believes this artificial limit to be the real limit. What we know of a living being is only a part of its real form. It is surrounded by the vapors it exhales, by radiations of great wavelength, which it is constantly emitting by reason of its temperature. Could our eyes see everything, a living being would appear to us as a cloud with changing contours.
Whence comes the crystal which appears in a solution? What is the starting point of the transformations undergone by the molecules of this solution before becoming a crystal? Observation shows that all living things from bacteria up to man, always proceed from an earlier being. Can it be the same with a crystal? Is it also derived by affiliation from an earlier being, or is it born spontaneously?
It seems now well proved, especially since the researches of Oswald, that with crystals both these modes of generation exist. In certain fixed conditions of the medium — of pressure, concentration of solutions, etc., liquids can only crystallize if they have first received a crystalline germ. The crystals thus formed may then, according to the expression of Dastre in his great work La Vie et la Mort, be considered as the posterity of an earlier crystal, absolutely in the same way that the bacteria developed in a solution represent the posterity of the bacteria originally introduced therein.
There exist, however, other conditions of the medium in which spontaneous crystallization may be observed without any previous introduction of germs. These different conditions being known and being producible at will, A solution may be placed either in conditions allowing it to crystallize spontaneously or in such that it will only crystallize after the introduction of suitable germs. It may therefore be said that crystals present two very distinct modes of reproduction — spontaneous generation and generation by affiliation.
This faculty of spontaneous generation, possible to the crystal being is, as is well known, impossible to the living being. The latter is only produced by affiliation, and never spontaneously. However, it must be admitted that before being born by affiliation, the original cells of the geological periods must have been born without parents. We are ignorant of the conditions which permitted matter to organize itself spontaneously for the first time, but nothing indicates that we shall always be thus ignorant.
We therefore see the notion accentuating that the crystal forms a being intermediate between brute and living matter, and placed nearer to the latter than to the former. It possesses in common with living beings the qualities above mentioned, and in particular something singularly resembling ancestral life. The crystalline germs we introduce into a solution in order to crystallize it seem to hint at a whole series of earlier lives. They recall the germs of living things — the spermatozoa which comprise the sum of the successive forms of a race, and contain, notwithstanding their insignificant size, all the details of the successive transformations which the living being exhibits before, arriving at the adult stage.
All the facts of this order belong to the category of unexplained phenomena of which nature is full, and which become more numerous as soon as we penetrate into unexplored regions. He complexity of things seems to increase the more they are studied.
Chapter IV: The Unity of the Composition of Simple Bodies
(1) Are the Different Simple Bodies Compounded From One Element?
When we submit the various compounds existing in nature to certain chemical operations, we succeed in separating them into elements which no reaction can further decompose. These irreducible elements are termed simple bodies, or chemical elements. From their combinations are formed our globe and the beings which inhabit it.
The idea that all bodies are supposed to be simple must be derived from one single element in different states of condensation or combination, come so naturally to the mind that it was put forth directly chemistry was established. After having been abandoned for want of proof, it was reborn when the recent experiments on the dissociation of matter seemed to show that the products resulting from the dissociation of the various bodies are formed of the same elements.
Facts known at an early date already indicated that the atoms of the most dissimilar bodies possessed certain properties in common. The most important of these are the identity of the specific heat and of the electric charge when, instead of with like weights of matter, we work with quantities proportional to the atomic weights.
Every one knows that the specific heat of bodies — the quantity of heat, expressed in calories, which has to be communicated to them in order to raise their temperature the same number of degrees — varies with different bodies. It is thus that, with the amount of heat necessary to raise a kilogram of water by 3°, the temperature of a kilogram of mercury can be raised by 97°. But if, instead of comparing equal weights of the different substances, weights proportional to their atomic weight are compared, it is noted that all bodies experience the same amount of heating from the same amount of heat, while electrolysis also proves that they carry an electric charge identical for the same atomic weight. To these facts, long known, are added those resulting from the recent researches here described, which show that, by the dissociation of matter, the like products are obtained from the most different bodies. It may therefore be admitted as extremely likely that all bodies are formed of one and the same element.
But even were the demonstration of this unity of composition complete, it would offer only a slight practical interest. By chemical analysis the same elements are discovered in a painting by Rembrandt as in a public-house signboard, and it is likewise proved that the body of a dog and that of a man have the same composition. Such observations as these give us absolutely no knowledge of the structure of the bodies thus analyzed. So far as atoms are concerned, what we desire to discover is the architectural laws which have enabled completely different edifices to be created with similar materials. Nothing is more probable than the fact that the atoms of chlorine, of zinc, and of the diamond are composed of one element. But how can this element give the elements of the various substances such different properties? Of this we are so completely ignorant that we are unable even to formulate any hypothesis on the subject.
Whatever may be the nature of the equilibria existing in the elements of the atoms of the various simple bodies, it is certain that these equilibria possess, in spite of their mobility, a very great stability since, after the most violent chemical reactions, the simple bodies are always again found unaltered. None of the transformations to which a given quantity of any element may be subjected modify either its nature of its weight. It is for this very reason that atoms have hitherto been considered indestructible.
This apparent indestructibility has always given great force to the belief in the invariability of chemical species. We shall see, however, that by looking a little closer into things, this argument loses much of its value; for, without involving the phenomenon of the dissociation of matter, we shall prove that the same bodies may really undergo very thorough transformations of properties, which sometimes even suggest actual transmutations.
(2) Can Simple Bodies be Considered as Elements of an Unvarying Fixity?
At the beginnings of chemistry the methods of analysis somewhat lacked refinement and the process of physical investigation, such as spectroscopy, were unknown. It was thus that arose with well defined properties. These bodies were too visibly different to be possibly confused. It was thus that arose the doctrine, analogous to that then admitted in biology, that chemical species were, like the species of living beings, invariable. Yet, after half a century of patient observation, biologists have finally abandoned the idea of the invariability of species, while chemists still defend it.
The facts discovered have shown, however, that there exists between chemical species as between Living species, transition at a good number of simple bodies by no means which cannot be disputed. It has had to be recognized that a good number of simple bodies by no means present clearly defined properties which allow them to be easily differentiated. There are many, on the contrary, so near to each other, possessing qualities so much alike, that no chemical reaction can distinguish them; and it was for this very reason that they were so long unknown. Almost a quarter of the simple bodies known — about 15, so resemble each other in their chemical characteristics that without the employment of certain methods of physical investigation (spectrum rays, electrical conductivity, specific heat, etc.) they could never have been isolated. These bodies are those metals the oxides of which form what are termed the “rare earths”. “They are only distinguished”, say M. Wyrouboff and Verneuil, “with but two or three exceptions, by their physical properties and are chemically identical. So much is this the case that no reaction has yet been found to separate them, and one is reduced, in order to obtain them in a more or less pure state, to the empirical and rude process of fractionation”.
Other recently discovered facts show that the most marked chemical species, such as ordinary metals, present numerous varieties. There exists, probably, round each element, a whole series of varieties with common characteristics, which possess, however, properties sufficiently sui generis for them to be distinguished; as is observed in living species. Silver, as we shall presently see, is not one single metal. There exist at least 5 or 6 kinds of silver, constituting very different simple bodies. It is the same with iron and, probably, with all the other metals.
The earlier chemistry carefully noted the existence of bodies seemingly identical in nature though differing in properties. It termed “allotropic” these different states of a same body. If it did not class them, as independent simple bodies, it was because by means of various reagents they could always be brought back to a common state. Red phosphorus differs entirely from white. And the diamond differs no less from carbon from carbon; but either white phosphorus or red can give the same compound — namely, phosphoric acid. With either coal or the diamond the same compound can also be made — namely, carbonic acid.
Without these common properties we should never have dreamed of classing together bodies so widely dissimilar as the coal and the diamond, or white and red phosphorus. White phosphorus is one of the bodies most greedy for oxygen and red phosphorus one of the least so. White phosphorus melts at 44° C, while red will not melt at any temperature and turns into vapor without passing through the liquid state. The first is one of the most poisonous bodies known, while the second is one of the most innocuous. Equally marked differences exist in greatly differing forms. M. Coste has shown that selenium slowly cooled is a good conductor of electricity, for which reason he has given it the name of metallic selenium. Ordinary vitreous selenium obtained by rapid cooling is, on the contrary, an insulator, and consequently no longer possesses the properties of a metal.
So long as the allotropic state was only observed in a very small number of bodies it was possible to look upon them as exceptions, but more sensitive methods of investigation have proved that what was considered exceptional constitutes, on the contrary, a very general law. The learned astronomer Deslandres supposes that the great differences observable in the spectrum of many bodies — carbon and nitrogen, for instance — according to the temperature at which they occur, are due to the allotropic states of these bodies” (Comptes Rendu, 14 Sept. 1903).
Without the need of invoking the hints supplied by spectrum analysis, it is very easy to note that the commonest and most distinguishable bodies, such as iron and silver, display many different allotropic states which allow us to class them as different species of the same genus. There are already half a dozen different kinds of iron and silver known which have clearly defined characteristics, although they possess certain reactions in common which formerly led to their being confused. It is probable that with new methods of observation the number of these species will be greatly increased. Recent researches on the colloidal metals, which we shall refer to in another chapter, are capable of being so modified as to lose all the properties of the metal from which they are derived and to resemble organic substances rather than metals.
But without even glancing at these extreme cases of colloidal metals, and only taking the most ordinary bodies, prepared by the absolutely classic methods, it has to be acknowledged, as we shall see, that the same metal can present itself in the forms impossible to be confused.
It is known that the heat absorbed or disengaged by the various simple bodies, in their combinations, is a constant quantity, represented by exact figures, and that it constitutes one of their essential characteristics. These figures, formerly considered invariable for each body, have served to found a special science — to wit, thermo-chemistry,
As soon as the allotropic forms of metals became known, these figures were taken in hand and it had to be acknowledged that, according to the mode of preparation of the metal, they might be 20 times higher or lower than the figures found for this same bodies when prepared by different methods. It cannot be said, for a great number of the figures published up to now, that they are even roughly approximate. It was Berthelot himself, one of the founders of thermo-chemistry, who contributed to the verification of this fact (1). It is very probable that had he done so 30 years earlier, thermo-chemistry would never have been born.
[(1) Here, moreover, are the figures obtained for silver by M. Berthelot according to the kind of metal employed — see Comptes Rendus, 4 February 1901. These figures represent the heat of the solution of an equal weight of substance in mercury:
(a) Silver in thin leaves, +2.03 cal
(b) Silver produced by the transformation of the above metal heated for 20 hr at 500-550 C in a current of oxygen, +0.47 cal
(c) Silver crystallized in needles; obtained by electrolysis from silver nitrate dissolved in 10 parts water: + 0.10 cal;
(d) Silver precipitated from its nitrate by copper, washed and dried, partly at the normal temperature: + 1.10 cal
(e) The above silver dried at 120 C: + 0.76 cal
(f) The above silver heated to a dark red: + 0.08 cal.]
From the standpoint taken by me as to the variability of chemical species, these results are of the greatest interest. From the standpoint of the ideas hitherto dominant on which thermo-chemistry was founded, they are plainly disastrous. M. Berthelot urges this by the following considerations:
“Such inequalities of energy as these being thus established by experiment, it is clear that there cannot be accorded with certitude to ordinary metals, nor, more generally, to elements; in the discussion of their reactions, the thermo-chemical values attained by starting from different states.
“The states of silver that I have studied do not, with one exception, answer to the figures of +7 cal for the heat of formation of the oxide Ag2O which is given in thermo-chemical treatises.
In the case of silver the thermo-chemical difference of the states of this element may rise, for one atom of silver, to 2 calories, which makes, for the formation of oxide, with 2 atoms of silver (AgO) a difference of +4 calories”.
The figures given in the books would then be, in the above case, wrong by nearly 50%. The same author then asks himself whether it might not be the same with iron, of which so many allotropic forms occur. The observation is probably applicable, not only to iron, but to all other bodies. What therefore is there left of all the figures which thermo-chemistry formerly displayed as so infallible?
There will probably remain very little, for even if we start from metals prepared in the same way, there is no certainty of starting from an identical body, since its simple dessication temperature permits its heat of combination to vary, and it is sufficient to very slightly change its physical state to also change its thermal properties. Faraday remarked that silver, deposited on a plate of glass by chemical means, had a great refracting power and a very feeble transparence. Faraday concluded from this that silver, in these two cases, must represent very different forms. And this prediction has been fully confirmed by experiment.
At the time when the figures of thermo-chemistry were established, chemists could not have reasoned other than they did, since they were not then able to differentiate bodies except by reactions incapable of bringing to light certain dissimilarities which were, however, fundamental. Silver, whatever its origin, when treated by nitric acid, invariably yielded silver nitrate of the same composition percent, and one could always extract from it the same quantity of metallic silver. How then was it possible to suspect that there existed in reality metals differing from each other, although representing the same appearance and known by the name of silver?
We nowadays know this because our methods of investigation have been perfected. When they are still more perfect, it is probable, as I have said before, that the number of chemical species derived from the same body will further increase.
The foregoing facts establish this important general law; that simple bodies are by no means composed of determined elements invariable in structure, but of elements which can be varies within rather wide limits. Every simple body only represents a type from which greatly different varieties are derived. B y adopting for the classification of metals that employed for living beings, it might be said that a metal like silver or iron constitutes a genus which includes several species. All the species of the same genus, the genus iron and the genus silver, for example, are absolutely distinct though possessing common characteristics. And if we consider that in the mineral world species are modified with some ease since, for instance, the white phosphorus species may become the red phosphorus species, or that the silver species, capable of disengaging many calories in its combinations, may become a species which disengages a smaller number, it is allowable to affirm that chemical species are much more easily transformable than animal species. It is not a matter for wonder, since the organization of the latter is much more complicated than that of the former.
Chemical species, then, are subject to variability. We know, on the other hand, that given certain appropriate actions, atoms may undergo the beginning of dissociation. May we hope, on the contrary, to succeed in totally transforming a simple body? This is the problem which we will now proceed to examine.
Chapter V: The Variability of Chemical Species
(1) Variability of Simple Bodies
“It is very rare” the celebrated chemist Dumas, “that one succeeds in comprehending the laws of a whole class of phenomena, by studying those whose action is displayed with the greatest intensity. It is generally the contrary which is observed, and it is nearly always by the patient analysis of a slight or slow phenomenon that one succeeds in discovering the laws of those which at first escaped analysis.
The whole history of science confirms this view. It was by attentively examining the oscillations of a hanging lamp that Galileo discovered the most important laws of mechanics. It was by a lengthened study of the shadow of a hair that Fresnel built up the theories which transformed the science of optics. It was by analyzing, with rudimentary apparatus, minute electric phenomena that Volta, Ampere, and Faraday called forth from the void a science which was shortly to become one of the most important factors in our civilization.
“It is certain that in the future as in the past”, writes Poincare, “the most profound discoveries, those which will suddenly reveal regions entirely unknown, and open up perfectly fresh horizons, will be made by a few men of genius who will pursue in solitary meditation their stubborn labor, and who, to verify their boldest conceptions, will doubtless require only the most simple and least costly methods of experiment”.
Considerations such as these should always be borne in mind by independent seekers when they find themselves stopped from want of means, or by the indifference or hostility which most often requites their labors. There exists, perhaps, no physical phenomena which, studied with patience in all its aspects, will not finally reveal, tanks to very simple means of investigation, totally unexpected facts. It was thus that the attentive study of the effluves generated by light on the bit of metal struck by it was the origin of all the researches noted in this work, and finally led me to demonstrate how little foundation there was for the century old dogma of the indestructibility of matter.
The great interest of such researches, when stubbornly followed up, consisted in constantly seeing new facts appear, and in never knowing into what unknown region one will be led. I have noticed this more than once during the many years devoted to my experiments. Undertaken with quite another object, they led me to study experimentally the question of the variability of chemical species; and if I give the preceding explanations, it is somewhat to excuse myself for having treated of a subject which would seem, at first sight, outside the scope of my researches.
From the philosophical point of view, the problem of the variability of chemical species is of the same order as that of the variability of the species of living organisms, which has for so long agitated science. Energetically denied at first, this variability of species has at last been accepted. The principal argument which led to its adoption is the extent of the variations to which beings can be subjects, although no one has ever succeeded in obtaining very great variations of some chemical species, the possibility of their transformation may be admitted for reasons of the same order as those which have appeared convincing to biologists.
The variability of chemical species, put in evidence in the preceding chapter by the simple statement of facts already known, needed to be first discussed in order to prepare the reader for the interpretation of the experiments I will now detail.
To obtain the transformation of certain bodies we shall require no energetic means, such as high temperature, great electric potential, or the like. I have already shown that matter, very resistant to mighty agencies, is on the contrary sensitive to slight excitants on condition that they are appropriate. It is precisely for this reason that, notwithstanding its stability, it can be dissociated under the influence of slight causes, such as a feeble ray of light.
I have already pointed out the very important part played by traces of a foreign substance when added to certain bodies. Its importance struck me as soon as I saw such curious properties as phosphorescence and such capital ones as radioactivity produced by the influence of such admixtures. If such important phenomena can be created by such very simple means, may it not be possible, by proceeding in an analogous manner, to succeed in modifying all the fundamental properties of certain elements?
By fundamental properties we understand those apparently irreducible ones upon which chemists rely for their classification. Thus, the property possessed by aluminum of not decomposing water when cold and of not being oxidized at the ordinary temperature constitutes one of the fundamental characteristics of this metal. If it can be compelled to oxidize water by simply adding to it traces of certain bodies, we shall evidently have the right to say that its fundamental properties have been modified.
As these experiments are merely accessory, since they go beyond the scope of my researches, I have only brought them to bear on three metals, namely aluminum, magnesium, and mercury. And as, although very simple, they necessitate certain technical explanations, I refer the reader for their detailed description to the purely experimental part of this work. It will there be seen that by putting the first two of these metals in the presence of traces of various substances — for example, distilled water which has served to wash out an empty flask previously containing mercury — it becomes possible to modify their characteristics that, if classified according to their new properties, their places in the list of elements would have to be altered. Thereafter, these metals, which are generally without any action on water, decompose it violently; the aluminum instantaneously becomes oxidized in air, becoming covered with thick tufts which grow under one’s eyes, and which give to a plate of polished aluminum the look of a jungle (See Bulletin de l’Institut Egyptien, Sec. 4, 19 November 1904, pp. 464 et seq.).
Several hypotheses were put forward to explain these facts when presented in my name to the Academie des Sciences. M. Berthelot pointed out that two metals in the presence of each other might form an electric couple which might be the origin of the phenomena noticed, and that, therefore, it would not be the properties of metals which were under observation but those of their couples. This is evidently a very insufficient explanation.
Other scholars have compared the metals this transformed to alloys which, according to certain ideas now in vogue, are constituted by combinations in defined proportions, dissolved in the excess of one of the metals in question. But in alloys, the changes obtained, such as hardness, fusibility, etc., are especially of a physical order, and in none of them are observed chemical transformations similar to those I have obtained.
By extending these researches, a large number of facts of the same order will certainly be discovered. Chemistry already possesses a certain number of them. There are, perhaps, as I have said, no bodies more dissimilar than white and red phosphorus. In certain of their fundamental chemical properties, amongst them their capacity for oxidation, they differ from each other almost as much as sodium from iron. Yet it is sufficient to add to white phosphorus traces of iodine or of selenium to transform it into red phosphorus.
The instances of iron and steel and of pure and ordinary iron are no less typical. It is known that steel, so dissimilar to iron in hardness and in appearance, only differs from it chemically by the presence of a few thousandth parts of carbon. It is also known that the properties of pure iron are absolutely different from those of ordinary iron. This last, in fact, does not oxidize in dry air. Pure iron obtained by reducing iron sesquioxide by means of heated hydrogen is so oxidizable that it spontaneously ignites in air, whence the name of pyrophoric iron given to it.
It might even be well, in the presence of such facts, to inquire whether the classic properties of several ordinary metals may not be solely due to some infinitesimal quantity of other bodies, the presence of which is often hidden from us, and which we call impurities when they are revealed to us by analysis. We shall see that the diastases, the most important compounds of organic chemistry, lose all their [properties when deprived of the traces of certain metals whose existence was formerly not even suspected.
The facts put in evidence by my researches and by those of the same order which I have brought together seem therefore to prove that simple bodies have not the invariability attributed to them. To admit that they are not invariable is to say that it may become possible to transform them, and to come back to the old problem of the transmutation of substances which so exercised the alchemists of the middle ages, and which modern science has finally judged to be as unworthy of its researches as the squaring of the circle or perpetual motion. Long considered as chimerical, it nowadays comes again to the front and occupies the minds of the most eminent chemists.
“The great modern discovery to be realized today”, wrote M. Moissan, “would not therefore be to increase by a single unit the number of our elements, but, on the contrary, to diminish it by passing in methodical fashion from one simple body to another… Shall we finally attain that transformation of simple bodies into one another which would play in chemistry as important a part as the idea of combustion when grasped by the acute mind of Lavoisier? Great questions here stand for solution. And this mineral chemistry, which we thought to be exhausted, is yet only at its dawn”. In reality, on the modern theory of electrolytic dissociation, chemists are obliged to admit, as everyday occurrences, transmutations quite as singular as those dreamed of by the alchemists, since it suffices to dissolve a salt in water to entirely transform its atoms.
It is known that, according to the theory even then old but greatly developed a few years ago by Arrhenius, in an aqueous solution of salt (potassium chloride, for example), the atoms of the chloride and potassium separate and remain present in the bosom of the liquid. Potassium chloride is dissociated by the sole fact of its solution into chlorine and potassium. But, as potassium is a metal which cannot remain in water without violently decomposing it, nor find itself in presence of chlorine without energetically combining with it, it must perforce be admitted that the chlorine and the potassium of this solution have acquired new properties bearing no analogy to their ordinary properties. It follows from this that their atoms have been entirely transformed. This is acknowledged, moreover, since the phenomenon is interpreted by the assertion that the differences noted are due to the fact that, in the solution, the atoms of chlorine and the atoms of potassium are formed of ions bearing electric charges of opposite signs, which would neutralize each other in ordinary chlorine and potassium. There must therefore exist two very different kinds of potassium, the potassium of the laboratory with all the properties we observe in it, and the ionized potassium without any relationship to the first; and the case is the same with chlorine. This theory has been accepted because it facilitates calculations, but it will be evident that it would lead us to consider the atom as the easiest thing in the world to transform, since it would suffice to dissolve a body in water in order to obtain a radical transformation of its characteristic elements.
Several chemists, moreover, formerly went some length in this direction. H. Sainte-Claire Deville declared to his pupils that he did not believe in the persistence of elements in compounds. W. Ostwald, Prof. of Chemistry at the University of Leipsic, likewise affirms that the elements cannot continue to subsist in chemical combinations. “It is”, according to him, “contrary to all evidence to allow that matter in a chemical reaction does not disappear and make room for another matter endowed with different properties”. Iron oxide, for instance, would nowise contain iron and oxygen. When oxygen is made to act on iron, a complete transformation is effected of the oxygen and iron, and if, from the oxide thus formed, oxygen and iron are subsequently extracted, it is only by performing the converse transformation. “Is it not nonsense”, writes M. Ostwald, “to claim that a definite substance can continue to exist without possessing any of its original properties? In point of fact, this purely formal hypothesis has only one object — that is, to make the general facts of chemistry agree with the utterly arbitrary notion of an unalterable matter”.
It certainly seems to result from what has been said above that the equilibria of the elements constituting the atoms can be easily modified, but it is indisputable also that they have an invincible tendency to return to certain forms of equilibrium special to each; since, after every possible modification, they are always able to return to their primary form of equilibrium. It may therefore be said that, in the present state of science, the variability of chemical species is proved, but that with the means at our disposal it is only realizable within certain limits.
(2) Variability of Compound Bodies
What I have just said of the variability of simple bodies and of the means which allow it to be effected applies equally to compound chemical bodies. There exists at the present day a very important industry — that of the manufacture of incandescent lamps — founded on nothing but the principle of the transformation of certain properties of compound bodies in the presence of slight quantities of other bodies. When the mantles of these lamps are soaked in pure thorium oxide, they do not become luminous on heating, or only very slightly so; but if the thorium oxide one percent of cerium oxide is added, the incandescence diminishes at once. This was a very unforeseen phenomenon, and is the reason why the creation of this mode of illumination required lengthy researches.
But it is, perhaps, in the chemical phenomena which occur in the interior of livin beings that this same principle can be more frequently verified. Divers diastases entirely lose their properties of they are stripped of the traces of mineral substances they contain, especially manganese. It is probable that bodies like arsenic, which is now extracted in infinitesimal doses from many tissues, exercise an important influence unsuspected by the earlier chemistry.
It is probably to the actions exercised by the presence of bodies in very small quantities that are due the differences observed in compounds formerly considered identical, which, however, would seem to vary with their origin. In former times well-defined radicals such as sugar, chlorophyll, hemoglobin, nicotine, the volatile essences, etc., were considered as identical, no matter from what living being they came. But Armand Gauthier has established that this is an error: “Though still appertaining to the same chemical family, these radicals, when isolated and closely studied, are modified from one vegetable race to another by isomerization, substitution, and oxidation: they have become, in short, other definite chemical species. It is the same with the animal kingdom. There is not one hemoglobin, but several hemoglobins, each proper to its own species”.
In noting these differences between bodies similar to each other, but of different origin, Gauthier does not give their causes. It is by analogy that I have supposed the said differences to be produced by traces of various substances, and by variations in their quantity. I have already pointed out that organic ferments lose their properties the moment they are deprived of the small proportion of metallic matter they always contain. Hemoglobin, which seems to act as a catalytic ferment, contains quantities of iron varying greatly with the animal species.
This principle of the transformation of the properties of a substance by the addition of a very small quantity of another body has thus plainly a general importance. Yet it is only the enunciation of empirical observations, of which the secret causes still remain hidden. The particular combinations thus formed, to which we shall return in a subsequent chapter, altogether escape the fundamental laws of chemistry.
The various applications I have made of this principle have proved to me that it will be fruitful and of practical use, not only in chemistry and physiology, but also in therapeutics. I base this assertion on some studies which I undertook several years ago on the totally new properties caffeine assumes when associated under certain conditions with very small doses of theobromine (an alkaloid which, when isolated, only acts on the organism in very large doses). From experiments made with registering instruments on various patients, several of which have been repeated in one of the laboratories of the Sorbonne by Prof. Charles Henry, theobromized caffeine would seem to be the most energetic muscular stimulant known. Observations made on a certain number of artists and writers have likewise proved its singular power on intellectual activity.
Experiments on the variability of compound chemical species have evidently not the same importance as those relating to the variability of simple bodies, since chemistry has for a long time known how to modify compound bodies by various reactions. If I have detailed them, it is to show that the principle of the method which permits the properties of simple bodies to be varied is applicable to many compound bodies, and to draw attention to its consequences in advance. In the early mineral chemistry, any compound bodies — silver nitrate, for instance — were considered as sharply defined substances formed by the combination of certain elements in strictly constant proportion. They are probably nothing of the kind. The law of definite proportions os no doubt only an approximate law like the law of Mariotte, and only owes its apparent correctness to the insufficiency of our methods of observation.
Insofar as the variability of simple bodies is concerned, it should be pointed out that a very serious reason, deduced from my researches, will no doubt always be opposed to the subjection of the atom to complete transformations of equilibrium. I have shown that it is a reservoir of colossal energy. It seems therefore probable that to transform it entirely would require quantities of energy far superior to those at our command.
But experiment proves that, without being able to definitely destroy the atomic equilibria, we are allowed to modify them. We know, also, that by very simple means we can provoke the dissociation of matter and consequently liberate a part of its energy. If, therefore, it is found impossible to add enough energy to the atom to transform it, we may at least hope to deprive it of a part of its energy, to cause it to go down a step which it cannot retrace in the scale of its successive steps. The atom deprived of a certain amount of energy can no longer be in the same state as before it lost it. Then it is, no doubt, that a veritable transmutation would appear.
Bringing together the facts above demonstrated we arrive at this conclusion. Matter, from which our experiments have banished immortality, no longer has the fixity attributed to it. It follows further that all the ideas still dominant on the invariability of chemical species seem sentenced to disappear. When we see how profound are the so-called allotropic transformations, the transformation of bodies in electrolytic solutions and the complete transformations of several metals in the presence of small quantities of certain substances; when too we see the facility with which bodies dissociate and reduce themselves to the same elements, we are naturally led to the renunciation of classical ideas and to the formulation of the following principle:
Chemical species are not invariable, any more than are living species.
Chapter VI: The Chemical Equilibria of Material Elements
(1) The Chemical Equilibria of Mineral Substances
The various elements may, by combination, give birth to bodies of an increasing complexity, from the minerals composing our globe up to the compounds forming the tissues of living beings.
For a long time chemistry has been studying these combinations. It might therefore be supposed that we are about to enter a very well-known field. A very short stay there will show that, on the contrary, it constitutes a world full of utterly unknown quarters.
As the mineral world was the only one accessible to the early methods of chemistry, it was naturally its first object of study. This was comparatively easy, and for this reason chemistry seemed at first a simple and precise science.
Mineral substances are, in fact, generally formed by combinations of a very small number of elements — oxygen, hydrogen, sulfur, etc. These combinations possess a constant composition and represent molecular edifices of small complexity in structure. It is only when we reach the compounds elaborated within the tissues of living beings that the phenomena become difficult to interpret. The molecular edifices then possess an excessive complication and a very great instability necessitated by the rapid production of energy requisite for the maintenance of life. The elementary edifice of the mineral world, composed of only a few stones, has becomes a town. The structure of organic substances sometimes reaches such a degree of complication that it very often escapes us altogether.
But however simple mineral edifices may appear, we are far from discerning the nature of the equilibria capable of giving them birth. It is solely the effects produced by these equilibria which are accessible to us. It is impossible for us to know wherein an atom of sulfur differs from an atom of oxygen or from any other form, and equally impossible to understand the cause of the different properties in the compounds formed by their combinations. All that can be said is, that the relative position of the atoms seems to determine the properties of bodies much more than the attributes supposed to be inherent in these atoms. There are hardly any properties of elements which one cannot manage to transform by modifying the structure of the molecular edifices in which they are united. What properties of the rigid diamond are found in the gaseous carbonic acid resulting from the combination of the diamond with oxygen? What properties of the suffocating chlorine, of the alterable sodium are met with in the sea salt formed by their association? Cacodyl and arsenic are very poisonous bodies, potassium a very caustic one; while potassium cacodylate of potassium, which contains 42% arsenic, is a body in no wise caustic and utterly inoffensive.
The properties of the elements then are capable of being entirely transformed by changes in the position of the atoms which enter into their structure. In chemistry, as in architecture, the shape of the edifice has a far greater importance than that of the materials which compose it.
It is principally the isomeric bodies — bodies possessing the same percentage of component parts though manifesting different properties, that is shown the importance of the structure of molecular edifices. In the isomeric bodies termed metameric there is not only the same proportional composition, but often the same number of atoms per molecule. The identity appears complete, but the difference in properties show that it cannot be so.
In bodies termed polymeric the percentage composition likewise remains identical, but the molecular weight varies by the condensation or by the splitting in two of the molecules. Such at least is the explanation given. If we could create polymeric elements from the metals we know we should probably succeed in creating new bodies, just as, by polymerizing acetylene by simply heating it, we transform it into benzene.
So long as chemistry had to handle only the very simple compounds of the mineral world —- water, acids, salts, etc., of which the composition was well known — it succeeded, by methodically varying their composition, in transforming their properties and in creating new bodies at will.
Take, for instance, as a combination with very little complication, the case of marsh gas or methane, which is composed of carbon and hydrogen (CH4). One can, by successively replacing an atom of hydrogen by an atom of chlorine, obtain very different products, such as mono-, bi-, tri-chlorinated (chloroform), or tetra-chloro-carbon (carbon tetrachloride).
All of these reactions, being very simple, can be expressed by very simple formulas. Had chemistry stopped at this phase, it might have been considered as a perfectly constituted science. The study of the chemical equilibria of organic substances has shown the insufficiency of the early notions.
(2) The Chemical Equilibria of Organic Substance
As soon as chemistry passed the bounds of the mineral world and penetrated into the study of the organic world, its phenomena became more and more complex. It was quickly noted that there existed equilibria independent of the percentage composition of bodies, and that consequently the customary formulas could not express them without giving the same formulas to very dissimilar bodies. It was necessary, therefore, to discard the early methods and to have recourse to geometrical figures, in order to approximately represent the structures coming to light. It was at first supposed — against all likelihood — that atoms ranged themselves on one plane according to geometrical lines, of which the hexagon was the type. Then it was at length understood that they were perforce disposed according to the three dimensions of space, and they then came to be represented by solid figure typified by the tetrahedron. Thus was born stereo-chemistry, which, without certainly telling us anything of the inaccessible architecture of atoms, permitted certain known facts to be put together and others to be discovered. But these diagrammatic structures, without any relationship to reality, in the long run showed themselves very insufficient. We were then led to suppose that the elements of bodies were not in static but in dynamic equilibrium. From this came a new chemistry, still in course of formation, which might be called kinematic chemistry. In its formulas atoms are represented by little circles, round which are drawn arrows indicating the supposed direction of their rotation. The idea that atoms and their component elements are in perpetual motion in bodies is quite in conformity with the notions I have set forth, but to interpret by diagram such complicated movements is evidently beyond our powers.
The most striking feature in the current conception is that chemical compounds appear more and more as mobile equilibria, varying with the external conditions such as temperature and pressure, to which they are subjected.
The reactions indicated by chemical equations owe their apparent rigidity only to the fact that the medium in which they are realized does not noticeably vary. When these conditions are much modified, the reactions immediately change and the usual equations are no longer applicable. What is called in chemistry the phase law was established through this fact being noticed. Any chemical combination ought always to be regarded as a state of equilibrium between the external forces which surround a body and the interior forces which it contains.
So long as chemistry had only to study very simple mineral or organic compounds elementary laws were sufficient, but closer examination showed that substances existed to which none of the known laws of chemistry could be applied, and these substances are just those which playa preponderating part in the phenomena of life. A living being is made up of an aggregate of chemical compounds formed by the combination of a small number of elements so associated as to compose molecular edifices of very great mobility. This mobility, necessary for the rapid production of a great quantity of energy, is one of the very conditions of existence. Life is bound up in the constant construction and destruction of very complicated and very unstable molecular edifices. Death, on the contrary, is characterized by the return to less complicated molecular edifices of very great stability of equilibrium.
A great number of chemical compounds of which the aggregate constitutes a living being, possess a structure and properties to which none of the old laws of chemistry are applicable. In this structure is sound a whole series of bodies — diastases, toxins, anti-toxins, alexins, etc., of which the existence has only, in most cases, been revealed by physiological characteristics. No formula can express their composition, and no theory explains their properties. On them depend the majority of the phenomena of life, and they possess the mysterious quality of producing very great effects without any apparent change in their composition and simply by their presence.
It is thus that the protoplasm which is the fundamental substance of the cells, never appears to change, although by its presence it determines the most complicated chemical reactions, notably those which result in the transformation of bodies containing energy at low potential into bodies whose potential is higher. The plant is able to manufacture, with compounds of small complication, such as water and carbonic acid, very complicated oxidizable molecular edifices, which are charged with energy. From the energy at low tension which surrounds it, it consequently manufactures energy at a high tension. It compresses the spring which other beings will relax to utilize its force.
The chemical edifices, which humble cells are able to form, comprise operations, not only the most skillful in our laboratories — namely, etherification, oxidation, reduction, polymerization, etc., but many more skillful still which we are unable to imitate. By means which we do not even suspect, the vital cells are able to construct those complicated and varied compounds — albuminoids, cellulose, fats, starch, etc., necessary for the support of life. They are able to decompose the most stable bodies, such as sodium chloride, to extract the nitrogen from ammoniacal salts, the phosphorus from phosphates, etc.
All these operations, so precise, so admirably adapted to one purpose, are directed by forces of which we have no conception, which act exactly as if they possessed a power of clairvoyance very superior to reason. What they accomplish every moment of our existence is far above what can be realized by the most advanced science.
A living being is an aggregate of cellular lives. So long as we are unable to comprehend the phenomena which take place in the bosom of an isolated cell, and have not discovered the forces which direct them, it will be of no use to build philosophical systems to explain life. Chemistry has, at least, achieved this much progress that it puts us face to face with a world of totally unknown reactions. For the former certainties of a too young science, it has finally substituted the uncertainties with which a more advanced science is ever burdened. They should not, however, be made too prominent, for the length of the journey before us would paralyze all efforts. Happily, those who enter upon these studies do not see how little advanced they are, and very often their teachers do not see it either. There is no dearth of learned formulas to conceal our ignorance.
What part may intra-atomic energy play in the reactions as yet so little known to us, which take place in the bosom of the cells? This is the point into which we will now inquire.
Chapter VII: Intra-Atomic Energy and the Unknown Equilibria of Matter
(1) Intra-Atomic Chemistry
I have just briefly demonstrated the existence of chemical actions which reveal certain equilibria of matter hitherto completely unknown. Without claiming to be able to determine the nature of these equilibria, will it not now be possible to more or less foreshadow their origin? It seems extremely probable that a large number of the inexplicable reactions we have mentioned, instead of only affecting molecular edifices, affect atomic edifices also, and bring into play the important forces of which we have proved the existence within them. Ordinary chemistry can displace the materials of which compounds are formed, but has not hitherto thought of dealing with these materials which it has considered to be indestructible.
Whatever interpretation may be given to the facts to follow, it is certain that they prove the existence of equilibria of matter which none of the early theories of chemistry could explain. We see in them important actions produced by reactions so slight that our balances cannot detect them, and phenomena which none of the doctrines of chemistry have foreseen, and which for the most part contradict them. We are on the threshold of a new science where our ordinary reagents and balances can be no help, since it is a question of reactions whose effects are enormous, notwithstanding that but infinitely small quantities of matter are brought into play.
The fundamental phenomena which reveal the dissociation of matter having been referred to elsewhere, it would be useless to go into the subject anew. The facts I am about to enumerate prove, in my opinion, that this dissociation has an important bearing on many phenomena hitherto unexplained.
These facts cannot be classed in any methodical fashion, since we have to do with a science yet unborn. I shall therefore confine myself to describing them in a series of paragraphs, without endeavoring to present them in the orderly manner which their fragmentary character does not allow.
(2) Colloid Metals
One of the best types of substances which elude the ordinary laws of chemistry is represented by the colloid metals. One of the methods of preparing them should alone suffice to indicate, apart from their very special properties, that their atoms must be partly dissociated. We have seen that, from the metallic poles of a static machine in motion there issue, as the result of the dissociation of matter, electrons and ions. Instead of a static machine let us take for the convenience of the experiment, an induction coil, the poles of which terminate in rods of the metal we wish to dissociate — gold or platinum for instance — which are plunged in distilled water. By making sparks pass between the two rods, as described by Bredig, a cloud will be seen to form round the electrodes/ After a certain time, the liquid becomes colored and contains, in addition to the metallic torn from the electrodes and proceeding from the dissociation of the metal. It is to this unknown thing that the name of colloid metal has been given. If the operation be long continued the colloid ceases to form, as if the liquid were saturated.
The properties of metals in a colloidal state are absolutely different from those of the body from which they emanate. In the prodigiously small proportion of 1/300 mg/liter, the colloid metal exercises a very energetic action which we will demonstrate later on.
The liquid in which the colloid metal is found is colored, but it is impossible to separate anything from it impossible to separate anything fro it by filtration, or to perceive in it with the microscope any particles, if they exist, are inferior in size to the wavelengths of light.
The ionic theory being applicable to most phenomena, it has naturally been applied to the colloids. A colloidal solution is today considered as containing granules bearing electric charges — some positive, the others negative. But whatever this rather too simple doctrine be worth, it is evident that a colloid metal has retained no traces of the same metal in the ordinary state. Its atoms have probably undergone a commencement of dissociation, and it is for this very reason that they no longer possess any of their former properties. Colloidal platinum or gold are certainly no longer either gold or platinum, though made from these metals.
The properties of colloid metals have, in fact, no analogy with those of a salt of the same metal in solution. By certain of their actions they resemble far more some organic compounds, notably the oxidases, than mineral salts. They present the greatest analogies with the toxins and the ferments, whence the name of inorganic ferments sometimes applied to them. Colloidal platinum decomposes oxygenated water as do certain ferments of the blood; it transforms alcohol by oxidation into acetic acid in the same way as does the mycodermina aceti. Colloidal iridium decomposes formiate of lime into calcium carbonate, carbonic acid, and hydrogen after the manner of certain bacteria. More curious still, bodies, which like prussic acid, iodine, etc., poison organic ferments, paralyze or destroy in the same manner the action of colloid metals.
The properties, at once at special and so energetic, of these metals led perforce to the study of their action on the organism, which is very intense. It is to their presence in various mineral waters that Prof. Garrigou attributes several properties of these waters — that of abolishing the phenomena of intoxication, for example. M. Robin has employed colloid metals as a remedy for sundry affections, notably typhoid fever and pneumonia, by injection. By injecting from 5 to 10 cubic milligrams of metal per liter. The result was a considerable increase of the organic exchanges, and of the oxidation of the elimination products as revealed by an over-production of urea and uric acid. These solutions being, unfortunately, very rapidly alterable, their practical use is very difficult.
There is, it will be seen, no relationship, close or distant, between the colloid metals and those from which they are derived. No chemical reaction can explain the properties they possess. Their mode of preparation authorizes the supposition that they contain, as I have said, certain elements of dissociated matter. I have, however, not observed in them any phenomena of radioactivity, but it will be readily understood that if these phenomena arise during the dissociation of matter, there is no reason for their appearance when matter is already dissociated.
Besides metals, many substances can exist in the state termed colloidal, and there is now a tendency to ascribe to this unknown form of the material equilibria a preponderant part in physiology. Protoplasm, for instance, would thus be only a mixture of colloidal substances — a fact, however, which throws very little light on its marvelous properties.
(3) The Diastases, The Enzymes, the Toxins, and Actions by Presence
To the colloidal metals obtained by the dissociation of various simple bodies must be compared the compounds classed under the name of diastases, toxins, enzymes, etc., whose reactions are near akin to those of the colloidal metals. Their chemical constitution is utterly unknown. They act almost exclusively by their presence and are sometimes extremely poisonous in almost imponderable doses. According to Armand Gauthier, two drops of the toxin of tetanus containing 99% of water, and 1% only of the active substance — which would hardly represent a milligram — is sufficient to kill a horse. A gram of this substance would suffice, he says, to kill 75,000 men. Such energies as these make one think of those which very slight atomic dissociations might manifest.
At the time when bacteria were believed to constitute the active agent of intoxications, it was possible to explain by their rapid multiplication the intensity observed in their effects, but it is now known that the toxins remain just as active after the bacteria have been separated by filtration. The living substance called yeast transforms glucose into alcohol and carbonic acid, but after having killed this yeast by heating it to a certain temperature, a substance can be extracted from it deprived of all organisms and called zymase, as capable of producing fermentation as the living yeast itself. The phenomena attributed a few years ago to microorganisms are therefore due to non-living chemical substances fabricated by them.
The part played by the various substances just mentioned in the phenomena of life is a very preponderant one. Most often it is only physiological reactions which reveal their existence and allow them to be isolated. All we know of them is that they lose their properties if deprived of the infinitely small quantities of mineral matters that they contain under a form that we suppose to border on the colloidal state.
Most of the above bodies — colloid metals, diastases, ferments, etc. — possess the property. Very inexplicable as yet, of acting, at least in appearance, by their presence alone. They do not appear in the products of the reactions which they excite. These actions of presence, also called catalytic, have been observed for a long time in chemistry. It was known, for example, that oxygen and sulfurous acid, though without action on one another, unite to form sulfuric acid in the presence of platinum black without the latter taking part in the reaction. So nitrate of ammonia, though ordinarily unalterable, also gives a continual disengagement of nitrogen in the presence of platinum black. This latter body does not combine with oxygen, but it can absorb 800 times its own volume of it. It is supposed — but this is evidently only a hypothesis — that it generally acts by borrowing oxygen from the air and conveying it to the substances with which it is in contact.
Among the substances of which one might strictly say that they act only by their presence is found the vapor of water, which in extremely small doses plays an important part in various reactions. Perfectly dry acetylene is without action on potassium hydride, but in the presence of a trace of humidity the two bodies react one on the other with such violence that the mixture becomes incandescent. Well-dried carbonic acid also is without action on potassium hydride, but in the presence of a slight quantity of steam it produces a formiate. It is the same with many other bodies — ammoniacal gas and hydrochloric gas, for example, which ordinarily combine with the emission of thick white fumes, but no longer do so after having been carefully dried. It will be remembered that I noted that by adding to dried salts of quinine traces of water vapor they become phosphorescent and radioactive.
Although catalytic actions were early known, it is only in the last few years that they have been proved to play a preponderant part in the chemistry of living beings. It is now admitted that the diastases and various ferments whose role is so important act only by their presence.
On closely examining the role of bodies acting by their mere presence, we note that they behave as if energy were transported from the catalyzing body to that catalyzed. This fact can hardly be explained, in my idea, unless by the catalyzing body undergoing the commencement of atomic dissociation. We know that, by reason of the enormous velocity possessed by particles of matter during its dissociation, considerable quantities of energy can be produced by the dissociation of a quantity of matter so imponderable as to elude all attempts to weigh it. The catalyzing substances should therefore be simply liberators of energy.
If this really be the case, we ought to be able to note that the catalyzing body at length undergoes a certain alteration. Now, this is exactly what is verified by observation. Platinum black and the colloid metals are in the long run worn out — by use they lose a great part of their catalyzing action.
(4) Oscillating Chemical Equilibria
All the reactions above indicated are, I repeat, inexplicable by current ideas. They are even contrary to the most important laws of chemistry, such as those of definite and of multiple proportions. We see, in fact, some bodies transform themselves under the influence of imponderable doses of certain substances, while others excite intense reactions by their mere presence, etc.
The study of early chemistry left on the mind the notion of very stable products, of well-defined and constant composition, and incapable of modification except by violent means such as high temperatures. Later on arose the notion of compounds less fixed, capable of receiving a whole series of modifications connected with the variations of the medium or of the temperature and of the pressure to which they are subjected. Of late years the notion has gradually arisen that any body whatever simply represents a state of equilibrium between the internal elements of which it is formed and the external elements acting upon it. If this connection is not plainly apparent in some bodies, it is because they are so constituted that their equilibria maintain themselves without perceptible changes within the limits of fairly large variations of the medium. Water can remain liquid in variations of temperature ranging from O° C to 100° C, and most metals do not appear to change their state within still wider limits.
It is now necessary to proceed farther and admit that outside the only factors till now regarded by chemistry — mass, pressure, and temperature — there are others in which occur the elements resulting from the dissociation of atoms. These elements should be capable of giving to bodies equilibria of such mobility that these equilibria could be destroyed or regenerated in a very short time under very slight external influences.
This succession of changes would be accompanied by the liberation of a certain quantity of the intra-atomic energy contained in matter. The actions by mere presence which are of such importance in the phenomena of life, may perhaps find an explanation in this theory. It was my studies of phosphorescence which led me to this hypothesis. It will be recollected that pure substances, various sulfides, phosphates of lime, etc., are never phosphorescent normally, and only become so when brought to a red heat for a length of time with traces of other various bodies — such as bismuth, manganese, copper, etc. I have shown, on the other hand, that this elevation of temperature always provokes a dissociation of matter. It is therefore permissible to suppose that the elements proceeding from this dissociation have an active part in the unknown compounds then formed, which gives to such bodies the capacity for phosphorescence.
The combinations thus obtained have precisely the characteristic pointed out above as belonging to extreme mobility — of destroying and regenerating themselves very rapidly. A ray of blue light falling on a screen of zinc sulfide, illuminating it in the tenth of a second, and a ray of red light falling on the same screen, destroys the phosphorescence in the same space of time — it brings the screen back to its primitive state. These two contrary operations, necessarily implying two converse reactions, may be indefinitely repeated.
However this may be, the facts enumerated in this chapter show us that chemistry is on the threshold of entirely new phenomena, characterized very probably by intra-atomic reactions accompanied by a liberation of energy. By reason of the enormous quantity of intra-atomic energy contained in matter, a loss of substance too small to be detected by our balances may be accompanied by a very great liberation of energy.
In endeavoring to bring the phenomenon of the dissociation of atoms into the explanation of unexplained chemical reactions, I have evidently only framed a hypothesis whose justification is not yet strong enough. It has at least the advantage of explaining facts hitherto without interpretation. It is certain that a phenomenon so important and frequent as that of the dissociation of matter must play a predominant part in many reactions. Intra-atomic energy is a science of which we barely see only the dawn. In this new science the old material of chemists, their balances and their reagents, will probably find their occupation gone.
Chapter VIII: The Birth, Evolution, and End of Matter
(1) Genesis and Evolution of Atoms
Barely 40 years ago it would have been impossible to write, on the subject I am now treating, a single line deduced from a scientific observation, and one might have thought that thick darkness would always envelop the history of the origin and development of atoms. How could they, moreover, be supposed to evolve? Was it not universally admitted that they were indestructible? Everything in the world changed and was ephemeral. Beings succeeded beings by assuming always new forms; stars were finally extinguished; but the atom alone did not submit to the action of time, and seemed eternal. The doctrine of its immutability reigned for 2000 years, and nothing allowed us to suppose that it might one day be shaken.
We have run through the experiments which have at last ruined this old belief. We now know that matter vanishes slowly, and consequently is not destined to last forever. But if the atoms are likewise condemned to a relatively ephemeral existence, it is natural to suppose they were not always what they are at the present day, and that they must have evolved during the succession of the ages. Through what successive phases have they passed? What forms have they step by step assumed? What were formerly the different substances we see around us — stone, lead, iron, in a word, all bodies? Astronomy alone could give some answer to such questions. Able to penetrate by spectrum analysis into the structure of the stars of various ages which illumine our nights, it has revealed to us the transformations to which matter is subject when it commences to grow old. We know that the spectrum analysis proves an incandescent body to have a spectrum reaching further towards the ultraviolet as its temperature rises. The same spectrum, moreover, has a maximum brilliancy which likewise moves towards the ultraviolet when the temperature of the luminous source rises, and towards the red when it diminishes. We know, on the other hand, that the spectral rays of a metal vary with its temperature. Watteville has even shown that if potassium be introduced into a flame, its spectrum changes according as the metal is in the more or less heated regions of this flame. The spectroscope gives us, then, the means of knowing from what elements the stars are composed, and how they vary with the temperature. In this manner it has been possible to follow their evolution.
The nebulae which show only the spectra of permanent gases like hydrogen, or products derived from carbon, must constitute, according to several astronomers, the first phase of the evolution of celestial bodies. By condensing they must form new stages of matter which end in the formation of stars. These latter represent very varying periods of evolution.
The whitest stars, which are also the hottest, as is proved by the prolongation of their spectrum into the ultraviolet, are composed of only a very small number of chemical elements. Sirius and alpha-Lyrae, for instance, contain almost exclusively incandescent hydrogen. In the red and yellow stars, stars less heated, which are beginning to cool and are therefore of great age, other chemical elements appear. First, magnesium, calcium, silicon, etc. Certain bodies are observed only in the coldest stars. It is therefore with the lowering of temperature that the elements of atoms undergo new phases of evolution, the result of which is the formation of certain simple bodies.
It is probable that the solid elements we observe — gold, silver, platinum, etc. — are bodies which have lost different quantities of their intra-atomic energy. Simple bodies in a gaseous state — nitrogen, hydrogen, oxygen — are the least numerous on our globe. To pass into a solid state, which they can only do at an extremely low temperature, they must first lose a very great amount of energy.
It seems very doubtful if heat is the sole cause of the sidereal evolution of the atoms. Other forces most probably have acted in it. We know that variations in pressure may, as Deslandres has shown, cause considerable variations in the rays of the spectrum; “under increasing pressures new series are seen to arise which only existed in germ at lower pressures”.
To sum up, the observation of the stars shows us the evolution of the atoms and the formation of the various simple bodies under the influence of this evolution.
We are ignorant of the nature and the mode of action of the forces capable of condensing a part of the ether which fills the universe into atoms of gas, such as hydrogen or helium, and then of transforming this gas into substances such as sodium, lead, or gold. But the changes observed in the stars are a proof that forces capable of effecting such transformations exist, that they have acted in the past, and that they continue to act in the present.
In the system of the world unfolded by Laplace, the sun and the planets were at first a great nebula, in the center of which was formed a nucleus animated by a rotary motion from which were successively detached rings which later on formed the earth and the other planets. Gaseous at first, these masses progressively cooled, and the space at first filled by the nebula was no longer occupied save by a small number of globes revolving on their own axes and round the sun. It is allowable to suppose that the atoms were not formed otherwise. We have seen that each of them may be considered as a little solar system comprising one or several central parts, round which revolve at immense speed thousands of particles. It is from the union of these miniature solar systems that matter is composed.
Our nebula, like all those still shining by night, must perforce have come from something. In the present state of science there is only, as far as we can see, the ether which can have constituted this cosmic starting point; and this is why all investigations always bring us back to consider it as the fundamental element of the universe. Worlds are born there and return thither to die.
We cannot say how the atom was constituted nor why it at length slowly vanishes, but at least we know that an evolution similar to this process pursues its way without halt, since we observe worlds in every phase of evolution from the nebula to the cooled planet, starting from suns still incandescent like our own. The transformations of the inorganic world now appear as certain as those of organized beings. The atoms, and consequently matter, do not escape that sovereign law which causes the beings which surround us and the innumerable stars with which the firmament is peopled, to be born, to grow, and to die.
(2) The End of Matter
I have attempted in this work to determine the nature of the products of the dematerialization of matter, and to show that they constitute by their properties substances intermediate between matter and the ether.
The ultimate term of the dematerialization of matter seems to be the ether in the bosom of which it is plunged. How does it return to it? What forms of equilibrium does it assume to affect this return? Here we are evidently on the extreme limit of the things our intelligence can comprehend, and are inevitably compelled to form hypotheses, but they will not be in vain if it be possible to give them precise facts and analogies for a support.
When studying the origin of electricity we saw that it might be regarded as one of the most f=general forms of the dematerialization of matter. We recognized, moreover, tha the final products of the dissociation of the radioactive bodies were formed of atoms of electricity. These last should therefore represent one of the last phases of the existence of material substances.
What is the fate of the atom of electricity after the dissociation of matter? Is it eternal while matter is not? If it possesses any individuality, how long does it keep it? And if it does not keep it, what becomes of the atom?
That the electric atom should be destined to have no end is very unlikely. It is on the extreme limit of things. If the existence of those elements had continued to exist, since their formation, under the influence of the various causes which produce the slow dissociation of matter, they would finally have accumulated to the extent of forming a new universe, or, at least, a kind of nebula. It is therefore likely that they at last lose their individual existence. But in what way, then, do they disappear? Are we to suppose that their destiny is that of those blocks of ice which float in the Polar regions, and which preserve an individual existence so long as the sole cause of destruction which can annihilate them — a rise in temperature — does not attack them? So soon as they are overtaken by this cause of destruction, they vanish into the ocean and disappear. Such, doubtless, is the final lot of the electric atom. Once it has radiated away all its energy, it vanishes into the ether and is no more.
Experiment furnishes a certain support to this hypothesis. I demonstrated with regard to the elements of dissociated matter emitted by the machines in our laboratories, that electric atoms in motion are always accompanied by vibrations of the ether. Such vibrations have received the names of Hertzian waves, radiant heat, visible light, ultraviolet light, etc., according to the effect on our senses or on our instruments, but we know tha their nature is the same. They may be compared to the waves of the ocean, which differ only by their size.
These vibrations of the ether, ever the companions of the electric atoms, most likely represent the form under which these vanish by the radiation of all their energy. He electric particle with an individuality of its own, of a defined and constant magnitude, would thus constitute the last stage but one of the disappearance of matter. The last of all would be represented by the vibrations of the ether, vibrations which possess no more durable individuality than do the waves formed in water when a stone is thrown into it, and which soon disappear.
How can the electric atoms proceeding from the dematerialization of matter preserve their individuality and transform themselves in vibrations of the ether?
All modern research leads is to consider these particles as constituted by whirls, analogous to gyroscopes, formed in the bosom of ether and connected with it by their lines of force. The question, therefore, reduces itself to this: how can a vortex formed in a fluid disappear into this fluid by causing vibrations in it?
Stated in this form, the solution of the problem presents no serious difficulties. It can be easily seen, in fact, how a vortex generated at the expense of a liquid can, when its equilibrium is disturbed, vanish by radiating away the energy it contains under the forms of vibration of the medium in which it is plunged. In this way, for example, a waterspout formed by a whirl of liquid loses its individuality and disappears in the ocean.
It is, no doubt, the same with the vibrations of the ether. They represent the last stage of the dematerialization of matter, the one preceding its final disappearance. After these ephemeral vibrations the ether returns to its repose, and matter has definitely disappeared. It has returned to the primitive ether from which hundreds of millions of ages and forces unknown to us can alone cause it to emerge, as it has emerged in the far-off ages when the first traces of our universe were outlined on the chaos. The beginning of things was, doubtless, nothing else than a re-beginning. Nothing leads to the belief that they had a real beginning, or that they can have an end.
If the views set forth in this work be correct, matter must have successively passed through very different stages of existence.
The first of these carries us back to the very origin of the worlds, and escapes all the data of experiment. It is the chaos epoch of ancient legends. What was to be one the universe was then only constituted of shapeless clouds of ether.
By becoming polarized and condensed under the influences of forces unknown to us, which acted through age piled on age, this ether was finally organized in the form of atoms, and it is from the aggregation of these last that matter as it exists in our globe or as we can observe it in the stars at various stages of their evolution, is composed.
During this period of progressive formation, the atoms have stored up the provision of energy they have to expend in various forms — heat, electricity, etc.— in the course of time. While thenceforth slowly losing the energy first stored up by them, they have undergone various evolutions and have consequently assumed varying aspects. Once they have radiated away all their store of energy in the form of luminous, caloric, or other vibrations, they return by the very fact of these consecutive radiations, to their dissociation— to the primitive ether whence they came. This last, therefore, represents the final nirvana to which all things return after a more or less ephemeral existence.
The evolution of the worlds would, therefore, in the last analysis, comprise two very different phases — one the condensation of energy into the atom, the other, the expending of this energy.
These brief sketches on the beginning of our universe and on its end evidently constitute only faint gleams projected into the deep darkness which envelopes our past and veils our future. They are doubtless very insufficient explanations, but science can as yet offer no others. It has not yet any glimpse of the time when it may discover the true first cause of things nor even arrive at the real causes of a single phenomenon. It must therefore leave to religions and to philosophies the care of imagining systems capable of satisfying our longing to know. All these systems represent the synthesis of our ignorance and of our hopes, and are consequently only pure illusions; but these creations of our dreams have always been more seductive than realities, for which reason man has never ceased to choose them as guides.
(3) Conclusions
The experiments analyzed in this work have allowed us to follow the atom from its birth to its decline. We have seen that matter, hitherto considered as indestructible, slowly vanishes through the dissociation of its component elements. This matter, formerly regarded as inert and as having only the power of giving back the energy which had been communicated to it, has, on the contrary, shown itself to us as an immense reservoir of forces. And from these forces are derived the majority of known modes of energy; molecular attractions, solar heat, and electricity in particular.
We have seen that matter can be dissociated under the influence of manifold causes, and that the products of its successive dematerializations constitute substances intermediate by their properties between matter and the ether. The result of this is that the ancient dichotomy between the world of the ponderable and that of the imponderable, formerly so widely separated, must disappear. And the study of the successive phases of the existence of matter has led us to the conclusion that the final term of its evolution is the return into the ether.
In thus endeavoring to catch a glimpse of the origins of matter, of its evolution and of its end, we have step by step arrived at the extreme limits of those semi-certitudes to which science can attain, and beyond which there is nothing but the darkness of the unknown.
My work is therefore finished. It represents the synthesis of laborious investigations carried on during many years. Starting with the attentive observation of the effects produced by light on a fragment of metal, I have been successively led by the concatenation of phenomena to explore very different fields of physics and to sketch in outline a synthesis of the universe.
Without doubt, experiment has always been my principal guide, but to interpret the results obtained and to discover others, I have had to set up more than one hypothesis. As soon as the obscure regions of science are entered, it is impossible to proceed otherwise. If you refuse to take hypothesis as a guide you must resign yourself to chance for your teacher. “The role of the hypothesis”, says Poincare, “is one which no mathematician can afford to ignore, any more than can an experimentalist”. To make hypotheses, to verify them by experiments, then to attempt to connect, by the aid of generalizations, the facts discovered, represents the stages necessary for the building up of all our knowledge.
In no other way have the great edifices of science been constructed. Imposing as they are, they still contain a large number of unverified theories, and it is often the least verifiable which play the greatest p[art in the direction of the researches of every epoch.
It is rightly said that science is the daughter of experiment, but it is very rare that experiment has not hypothesis for its guide. This last is the magic wand which evokes the known from the unknown, the real from the unreal, and gives a body to the most shadowy chimeras. From the heroic ages down to modern times, hypothesis has always been one of the mainsprings of the man’s activity. It is by religious hypotheses that the most imposing civilizations have been founded, and it is with scientific hypotheses that the greatest modern discoveries have been accomplished. Modern science accepts them no less than did our forefathers — and their role is, in reality, much greater now than ever it was, and no science could progress without their aid.
Hypotheses above all serve to found those sovereign dogmas which occupy, in science, as preponderant a part as in religions and philosophies. The learned just as much as the ignorant man, has need of faith to give direction to his researches and to guide his thoughts. He can create nothing if not animated by some faith, but must not remain too long unmoved in that faith. Dogmas become dangerous so soon as they commence to grow old.
It matters little that hypotheses and the beliefs they generate be insufficient; it is enough that they are fruitful, and they become so as soon as they provoke research. Strictly verifiable hypotheses do not exist. Neither do absolutely positive laws. The most important of the principles on which all the sciences rely are only truths approximately true within certain limits, but which, outside those limits, lose all exactitude.
Science lives on facts, but it has always been great generalizations which have given them birth. A fundamental theory cannot be modified without the direction of scientific researches at once changing. From the single fact that ideas on the constitution and invariability of atoms are in course of transformation, the doctrines which once formed a basis for the foundations of physics, of chemistry, and of mechanics, together with the direction of research, will have to change likewise. This new orientation in research will necessarily bring with it an outburst of new and unexpected facts.
No one could dream of studying the world of atoms at the still recent time when they were thought to be formed of simple, irreducible, inaccessible, and indestructible elements. Today we know that science is able to attack these elements, and that each one of them is a small universe of an extraordinarily complicated structure, a repository of forces formerly unknown, the magnitude whereof exceeds enormously all those hitherto known. That which chemistry and physics believed they knew best was in reality what they knew least.
It is in these atomic universes, whose nature was so long misunderstood, that must be sought the explanation of most of the mysteries which surround us. The atom, which is not eternal; as the ancient creeds asserted, is far more powerful than if it were indestructible and therefore incapable of change. It is no longer a thing inert, the blind sport of all the forces of the universe. It is the very soul of things. It stores up the energies which are the mainspring of the world and the beings which animate it. Notwithstanding its infinite minuteness, the atom perhaps contains all the secrets of the infinite greatness.